JBRA Assist. Reprod. 2015; 19 (2):66-69
ORIGINAL ARTICLE
doi: 10.5935/1518-0557.20150015
Annexin V-MACS in infertile couples as method for separation of sperm without DNA fragmentation
1Centro de Fertilidad y Reproduccion Asistida (CEFRA), Lima- Peru.
REDLARA AWARD Best Oral Presentation. 12º REDLARA General Congress - Lima - Peru 2015
CONFLICT OF INTERESTS
No conflict of interest have been declared.
ABSTRACT
Objective: To determine the effect of using MACS technology on clinical pregnancy, as a method for separation of damaged sperm in infertile patients.
Methods: 136 infertile men having normal semen parameters in accordance with WHO 2010 criterion, undergoing ICSI cycle were enrolled during the course of the study. The patients were prospectively randomized and enrolled after oocyte retrieval and were assigned to the ICSI group, PICSI group or MACS group. Embryo development and clinical pregnancy were assessed. In 17 randomized MACS patients, sperm DNA fragmentation was tested in the presumptive apoptotic and no apoptotic spermatozoa fractions.
Results: Similar results were obtained between groups for the following parameters: fertilization rates of 78.97% (95% confidence interval [CI]:74.37-83.57), 70.15 %(95% CI:63.98-76.33) and 80.28%(95% CI:73.74-86.81) for ICSI, PICSI and MACS group, respectively; Number of Day-3 embryos was 5.04 (95% CI:4.09-5.98), 5.17(95% CI:4.24-6.10) and 5.59(95% CI:4.31-6.87) for ICSI, PICSI and MACS group, respectively; number of freezing embryos in blastocyst stage was 0.78 (95% CI:0.25-1.31), 0.70(95% CI:0.27-1.14) and 1 (95% CI:0.37-1.6) for ICSI, PICSI and MACS group, respectively. However, clinical pregnancy rates of 58.1% for MACS group versus 40.4% and 27.3% for PICSI and ICSI group, respectively, were showed statistical difference (P=0.019). DNA fragmentation index for the two sperm MACS fraction showed statistical differences (P=0.000), MACS reduced the D.F.I of the sperm sample.
Conclusions: The use of MACS technology improves the clinical pregnancy on infertile couples and can be applied as a method for sperm separation, discriminating sperm with high DNA fragmentation.
Keywords: MACS, ICSI, DNA fragmentation, PICSI, Morphology.
INTRODUCTION
Selection of human spermatozoa prior to intracytoplasmic sperm injection (ICSI) technique is currently based on criteria as viability, motility and morphology. Double density gradient centrifugation (DGC) and the swim-up procedure are currently used as standard preparation techniques. However, new insights in the development of molecular selection strategies have been introduced: Namely, the hyaluronic acid (HA) mediated sperm selection know as Physiologic ICSI (PICSI) and the annexin V magnetic activated cell separation (MACS) are ones of the most recently methods introduced (Paasch et al., 2007).
MACS is a new tool to optimize sperm selection in assisted reproduction. The technique is based on the binding of superparamagnetic Annexin-microbeads to externalized phosphatidylserine (PS) at the outer leaflet of the plasma membrane of sperm with activated apoptosis signaling or membrane damage (Grunewald & Paasch, 2013; Sheikhi et al., 2013). There are already some reports comparing ICSI with MACS. However the positive effect of MACS sperm selection is still to be confirmed in larger-scale studies; in the meantime, our study compare the clinical pregnancy and embryo development in MACS, with PICSI and conventional ICSI.
MATERIAL AND METHODS
Design
It was a unicentric, prospective and randomized study at the Centro de Fertilidad y Reproduccion Asistida (CEFRA), Lima- Peru. The main endpoint of this study was clinical pregnancy after ICSI, with and without use of MACS, PICSI or conventional sperm selection method.
Patient
From January 2013 to July 2014 patients with infertility having normal sperm concentration parameter in accordance with WHO 2010 criterion, undergoing ICSI cycle were enrolled during the course of the study. 136 patients were prospectively randomized after oocyte retrieval and were assigned to either the ICSI group (for which sperm selection for injection was only based on visual assessment), PICSI group, or to MACS group. The endometriosis was the unique exclusion criteria used in women.
Procedure
Oocyte cumulus complexes (OCC’s) were retrieved in buffered media (GlobalW/Hepes, Life global, USA) supplemented with 5% of Human Seroalbumin (HSA) and transferred to Global Total for Fertilization (Life Global, USA) for culture at 37°C, 5% O2 and 6% CO2 for 3 to 4h. Meanwhile, the semen sample (obtained by masturbation from the husband) was subjected to double density gradient centrifugation (All Grade, Life Global, USA) at 300 g for 10 min. The resulting pellet was washed (Global W/Hepes, Life Global, USA) and allocated to any of the groups of sperm selection.
OCC’s were denuded mechanically using a glass pipette after brief exposure to hyaluronidase (Hyaluronidase, Life Global, USA) for 30s. Mature oocytes were injected with sperm according to the standard ICSI protocol of our center. In the ICSI group, a 5μl polyvinyl pyrrolidone drop (PVP, Life Global, USA) under oil (Lite Oil, LifeGlobal, USA), was used to select spermatozoa with normal morphology for subsequent injection. In the PICSI group, SpermSlow™ (MediCult–Origio) was applied in order to get the most viable sperm, the dish for sperm injection was prepared according to the protocol used by Parmegiani et al., 2012. In the MACS group, the pellet was resuspended in 2–5 ml with 1X binding buffer (BB; Miltenyi Biotec, Germany), centrifuged at 300g for 5 min and the supernatant removed. The sample was then incubated with annexin V conjugated microbeads (Miltenyi Biotec) for 30min at 25°C during continuous agitation. After rinsing the column with BB, the sperm-bead suspension was added on top with BB and, immediately after that, the annexin V-negative fraction was obtained. To obtain the annexin V-positive fraction, the column was taken out of the magnet and 1 ml BB was added to rinse it using a plunger provided by the manufacturer (MiniMACS; Miltenyi Biotec).
The fraction of presumptive no apoptotic fraction with intact membranes were used for ICSI. Both fractions were stored in order to evaluate sperm DNA fragmentation.
In order to evaluate the MACS system we performed Sperm chromatin dispersion test (SCDt) with the Halosperm kit (Halotech DNA, Spain) in both fraction accordance to the MACS system: apoptotic fraction and no apoptotic fraction in 17 patients chosen at random.
DNA Fragmentation Index (D.F.I) was recorder in order to compare in both fractions (Fernandez et al., 2003)
Fertilization was checked the next morning at 16–18h post insemination checking the presence of 2PN; embryos were then prolonged cultured in 50 ul drops of Global Total Medium (Life Global, USA) covered with mineral oil (Lite oil, Life global, USA) from day 1 to 3 and from day 3 to 5. Embryo transferred was performed on Day-3 or Day-5 post injection depending of the embryologist scoring/decision, and extra embryos were vitrified on Day-5 if blastocyst was found.
Fertilization rate was calculated as the ratio of number of 2PN embryos over the total number of MII oocytes injected, and expressed as percentage.
A clinical pregnancy was defined as presence of one or more gestational sacs with heart beat on ultrasound 2 to 3 weeks after positive ßhCG. Ectopic pregnancies were not counted as clinical pregnancies.
Pregnancy loss rate was defined as the number of clinical pregnancies lost before 20 weeks of gestation divided by the total number of clinical pregnancies.
Statistical Analysis
Data were checked for normality before statistical analysis. To compare the various groups and to make the comparisons normally distributed continuous variables were compared using Post hoc test Benforrini, while Kruskal – Wallis tests were performed to evaluate differences in the median for those variables that were not normally distributed.
Categorical variables were analyzed using the chi square test. t-student was applied for related samples.
For all statistical tests, a P- value lower than 0.05 was considered statistically significant.
RESULTS
Out of 136 patients that were enrolled and randomized for the study, 3 cases from the MACS group were excluded from the pregnancy rate analysis since they did not have a embryo transfer instead all embryos were frozen. A total of 55 cases were analyzed in the ICSI group, 47 cases were analyzed in the PICSI group and 34 cases were analyzed in the MACS group. There were no significant differences in female age, male age, BMI, semen volume, sperm concentration and motility sperm (Table 1).
The number of oocytes and MII’s retrieved, injected, the number of Day-3 embryos, the number of embryos frozen and the number of embryos transferred between the three groups showed no significance differences (Table 2). Similarly, no differences in the number of 2PN were observed; however, the fertilization was reduced in 8% in the PICSI Group compared to others (P= 0.036), and no differences between other groups were observed. The number of Day-3 embryos, number of freezing embryos was similar in all the groups and no significant differences were found; the number of embryos transferred was similar in ICSI, PICSI and MACS group (2.18, 2.26 and 1.94, respectively). The clinical pregnancy rate was statistical significantly higher in the MACS group compared to ICSI and PICSI groups (58.1%, 27.3 % and 40.4 % respectively; P= 0.019). Biochemical pregnancy and pregnancy loss rate was not significantly different between the three groups (Table 2).
To further validate the efficiency of MACS technique for sperm separation, we performed sperm DNA fragmentation analyses in 17 patients. There was a significant reduction of sperm with fragmented DNA after MACS system (P= 0.000). Furthermore, from the 17 patients 10 who got pregnant, the D.F.I was less than 14% in the samples used for ICSI (Table 3).
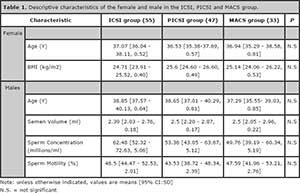
Table 1. Descriptive characteristics of the female and male in the ICSI, PICSI and MACS group.
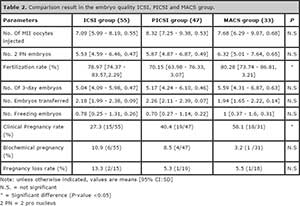
Table 2. Comparison result in the embryo quality ICSI, PICSI and MACS group.
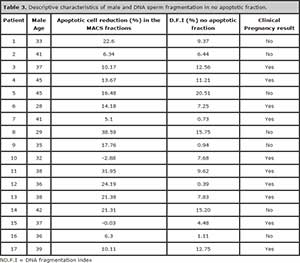
Table 3. Descriptive characteristics of male and DNA sperm fragmentation in no apoptotic fraction.
DISCUSSION
The selection of ideal spermatozoa before ICSI could help to optimize the outcome of the treatment, because the injection of a sperm based on viability and normal morphology does not eliminate the chance for damaged DNA or immature spermatozoa and may account for a considerable percentage of failed embryo development post-ICSI. Furthermore, infertile men with spermatozoa with apparently normal morphology may have DNA fragmentation (Avendaño & Oehninger, 2011).
Different laboratories have introduced novel procedures to replace traditional sperm selection methods, however, the efficiency of these techniques is still debatable and questionable (Nasr-Esfahani et al., 2012).
The use of magnetic cell sorting using annexin V-conjugated micro beads, at molecular level can mark and isolate sperm with PS translocation to the membrane, specifically targets spermatozoa with damaged membranes, which is a manifestation of apoptosis. However, there is not enough information concluding the benefits of these methods. In fact, Romany et al. (2014) applying MACS technology in unselected males did not find an improvement in the reproductive outcome of ICSI in ovum donation cycles. In our case we decided to test the benefit of MACS in infertile couples because we hypothesize the benefit of avoiding oocyte quality bias could have a disadvantage, since the oocyte can repair the DNA strand breaks before the initiation of the first cleavage division. It is well know that recognition of DNA lesions and repair in the paternal chromatin after fertilization by the oocytes (Derijck et al., 2008; Barton et al., 2007; Ashwood-Smith & Edwards, 1996) and the capability to DNA repair of the oocytes are age depended. In fact, we found significant differences in clinical pregnancy compared to ICSI group (58.1% vs 27.3 %, respectively). Biochemical pregnancy and pregnancy loss are reduced in MACS groups, however they were not statistical significant (Table 2).
Favoring selection of spermatozoa with intact DNA and normal nucleus, HA may be another alternative to optimize ICSI outcome, in fact some studies have shown significant differences (Parmegiani, 2010) while others did not find differences in clinical pregnancy rate or embryo quality (Ménézo & Nicollet, 2004; Majumdar & Majumdar, 2013). We decided to compare the two new methods for sperm selection, patients of PICSI group show clinical pregnancy rate benefits compared to ICSI group (40.4% vs 27.3 %, respectively), but its benefit was inferior to the MACS group (58.1%). It was remarkable to observe a reduced pregnancy loss in both method of sperm selection, PICSI and MACS (Table 2).
We can conclude that ICSI could be improved applying another sperm selection method, MACS would be an alternative because it can limit the amount of selected sperm (for ICSI) with damaged DNA ultimately leading to improved clinical pregnancy rates.
Acknowledgements:
To Dra. Patricia Orihuela, Dr. Francisco Escudero, Dr. Ygor Perez , Dr. Mario Garcia , Dr. Remo Angeles , Blg. Ricardo Pella for their contribution and the andrology and embryology team of CEFRA. Finally, to Sergio Romero PhD. for his help in manuscript review.
REFERENCES
Avendaño C, Oehninger S. DNA fragmentation in morphologically normal spermatozoa: how much should we be concerned in the ICSI era? J Androl. 2011; 32:356-63.
Medline Crossref
Ashwood-Smith MJ, Edwards RG. DNA repair by oocytes. Mol Hum Reprod. 1996; 2:46-51.
Medline Crossref
Barton TS, Robaire B, Hales BF. DNA damage recognition in the rat zygote following chronic paternal cyclophosphamide exposure. Toxicol Sci. 2007; 100:495–503.
Medline Crossref
Derijck A, van der Heijden G, Giele M, Philippens M, de Boer P. DNA double-strand break repair in parental chromatin of mouse zygotes, the first cell cycle as an origin of de novo mutation. Hum Mol Genet. 2008; 17:1922–37.
Medline Crossref
Fernandez JL, Muriel L, Rivero MT, Goyanes V, Vasquez R. Alvarez JG. The Sperm Chromatin Dispersion Test: A Simple Method for the Determination of Sperm DNA Fragmentation. J Androl. 2003; 24:59-66.
Medline
Grunewald S, Paasch U. Sperm selection for ICSI using annexin V. Methods Mol Biol. 2013; 927: 257-62.
Medline Crossref
Majumdar G, Majumdar A. A prospective randomized study to evaluate the effect
of hyaluronic acid sperm selection on the intracytoplasmic sperm injection outcome of patients with unexplained infertility having normal semen parameters. J Assist Reprod Genet. 2013; 30:1471–5.
Medline Crossref
Nasr-Esfahani MH, Deemeh MR, Tavalaee M. New era in sperm selection for ICSI. Int J Androl. 2012; 35:475-84.
Medline Crossref
Romany L, Garrido L, Motato Y, Aparicio B, Remohí J, Meseguer M. Removal of annexin V–positive sperm cells for intracytoplasmic sperm injection in ovum donation cycles does not improve reproductive outcome: a controlled and randomized trial in unselected males. Fertil Steril. 2014; 102:1567-76.
Medline Crossref
Paasch U, Grunewald S, Glander HJ. Sperm selection in assisted reproductive techniques. Soc Reprod Fertil Suppl 2007; 65:515–25.
Medline
Parmegiani L, Cognigni GE, Bernardi S, Troilo E, Ciampaglia W, Filicori M. ‘‘Physiologic ICSI’’: Hyaluronic acid (HA) favors selection of spermatozoa without DNA fragmentation and with normal nucleus, resulting in improvement of embryo quality. Fertil Steril. 2010; 93; 598-604.
Medline Crossref
Parmegiani L, Cognigni GE, Bernardi S., Troilo E, Taraborrelli S, Arnone A, Maccarini A.M., Filicori M. Comparison of two ready-to-use systems designed for sperm–hyaluronic acid binding selection before intracytoplasmic sperm injection: PICSI vs. Sperm Slow: a prospective, randomized trial. Fertil Steril. 2012, 98:632–7.
Medline Crossref
Sheikhi A, Jalali M, Gholamian M. Elimination of apoptotic spermatozoa by magnetic-activated cell sorting improves the fertilization rate of couples treated with ICSI procedure. Andrology. 2013; 1: 845-9.
Medline Crossref