JBRA Assist. Reprod. 2016;20 (3):107-111
ORIGINAL ARTICLE
doi: 10.5935/1518-0557.20160025
A descriptive study of culture media in Brazilian assisted reproduction clinics
1Assisted
Reproduction Center - Department Gynecology and Obstetrics – Medical
School – University of Ribeirão Preto (UNAERP), Ribeirão Preto/SP -
Brazil
2Human Reproduction Center of the Ana Bartmann Clinic - Ribeirão Preto/SP – Brazil
3Universidade Federal de Alfenas – MG- Brazil
CONFLICT OF INTERESTS
No conflict of interest have been declared.
ABSTRACT
Objective:
The present study aimed to draw a profile of the most commonly used
media and protocol characteristics from assisted reproduction
technology (ART) facilities in Brazil.
Methods:
To obtain an overview of ART methods and culture media, a questionnaire
was given to embryologists from ART clinics in Brazil. Further research
in scientific papers and journals was carried out for describing the
processes around Brazil, USA and Europe.
Results:
From the questionnaire, we found that the embryo medium mostly used is
CSCM™ from Irvine Scientific, represented 37.04% in Brazilian ART
clinics; interestingly, 70.37% of clinics exchange the embryo media
bath; however, 70.37% do not change the media type. Transfers in
Brazilian clinics were variable, but day 3 transfer was a procedure
seen in 37.04%. The remaining embryos are habitually maintained in
prolonged cultivation in 51.85% of the clinics interviewed.
Conclusion:
Although there are numerous studies trying to better understand embryo
culture media influences, there is a lack of evidence for choosing one
as the most appropriate. In short, it is a random decision for such an
essential stage of In Vitro Fertilization.
Keywords: Culture media, Embryo transfer, Brazilian clinics.
INTRODUCTION
Infertility is an important public health issue affecting lives
worldwide. Since 1978, when the first IVF baby was born, over 5 million
infants have resulted from assisted reproduction technology (ART) (ESHRE, 2014).
Multiple steps are taken in an In Vitro Fertilization (IVF) treatment
cycle; including ovarian stimulation, oocyte retrieval, fertilization
in a liquid medium, embryo selection and embryo transfer into the
uterine environment (Sicignano et al., 2010).
Understanding not only protocols and techniques, but also the products
used for IVF is essential for a successful ART course. In short, the
fertilization media are crucial for thriving IVF processes.
There
are ART regulations worldwide as well as several federal laws in
Europe, USA and Brazil. In these three places, there are regulations
regarding the ART data from each clinic. Following this information
governed by laws, some differences between IVF processes are easily
seen. The number of clinics and egg transfer quantity are good
examples. The reported clinics in Europe were 1,314 in 33 countries, in
2011(European IVF-Monitoring Consortium et al., 2016); in USA, there were 467 clinics in 2013 (Centers for Disease Control and Prevention et al., 2015); and in Brazil, 106 by regulated data, however, a total of 130 clinics was estimated for 2014 (ANVISA, 2015). The number of egg transfers in Europe was 367,171 (European IVF-Monitoring Consortium et al., 2016); 73,571 in USA (Centers for Disease Control and Prevention, 2015); and in Brazil, 60,668 (ANVISA, 2015).
Despite these dissimilar numbers, advances in IVF during the last
decades have been rapid and impressive; nevertheless, culture media is
estimated to play a major role in this success (Chronopoulou & Harper, 2015). Beyond the types of media, the differences can be seen in protocols for preparing and using each specific medium.
A scientific and educational institution brings together more than 90% of the ART centers in Latin America (REDLARA, 2016)
named RED – Latin American Register of Assisted Reproduction, published
the “Manual of Procedures – Assisted Reproduction Laboratory” in 2006.
This manual reveals that some ART centers have preferred to prepare
media in their own laboratory, in spite of knowing the media simplicity
prepared in laboratory and the importance of its quality for embryo
development (REDLARA, 2006).
There are many studies offering comparisons between methods of cultivation (Marianowski et al., 2007), which analyses open culture and closed culture or that compare media with gene expression (Kleijkers et al., 2015). Therefore, the descriptive tone of this paper justified as a means to complement this field of study.
Although many studies have been carried out about embryo culture media, a recent review (Youssef et al., 2015) concluded for the lack of evidence to support or refute the use of any specific culture medium.
MATERIAL AND METHODS
a. Study design and settings
This study involved a cross-sectional evaluation of the most used IVF
media in ART clinics in Brazil, discussing data from papers and the
latest governmental databases from USA and Europe through searches in
the NHS and Google Scholar, published between 1978 and January 2016. In
addition, we collected data from Scielo and Brazilian government
databases. The search for papers was done using keywords such as
“cultivation media”, “embryo cultivation media”, “IVF cultivation
media”, “embryos cultivation”, “culture media” or “culture media for
embryos”.
b. Sampling and eligibility
A
contact list was created within 55 randomized ART clinics in Brazil. To
obtain as much information as possible, we contacted only the clinics
working with ART. Embryologists from those clinics were contacted via
e-mail or phone. In such case, embryologists answered a simple
questionnaire in January 2016, and the clinics were enrolled into the
study. Those who were not willing to be contacted and those who did not
respond were excluded from the contact list.
c. Recruitment, enrolment and instrumentation
An initial contact letter was mass-e-mailed to ART working
embryologists on the contact list. The first contact aimed to discuss
the study and the embryologists were asked to complete and e-mail the
questionnaire enclosed. At the closing date of the survey, 27 (49%)
embryologists had not replied to the initial letter, and 28 (51%)
refused to participate or did not answer the questionnaire, leaving 27
(49%) to participate in the research.
The survey instrument was entirely focused on assessing embryologists
working in ART clinics, with simple questions [Fig 1], but sufficient
to elucidate the different characteristics in protocols. A 5-items
self-administered questionnaire was used to collect data on: the
culture media used in the services; bath exchanging and media changing
– for sequential media; transfer days usually used; remaining embryos
destination, and if cryopreserved or kept in prolonged cultivation.
In Brazil, the culture media available are from: Irvine Scientific™,
Cook™, Vitrolife, LifeGlobal, Ingámed, Origio and Sage. Here we shall
focus exclusively on the media that appeared as an answer in the
questionnaire.
Concerning the exchanging of bath, we aimed to know if the media
culture was renewed, meaning the total removal of the first culture
media without changing the bath type. For the question of changes in
media, the aim was to know if the media type was changed to another
brand or even to a different type from the same brand. In relation to
transferring day and supernumerary embryos, the objective was to
describe the different protocols followed by clinics in Brazil. The
nomenclature of days was done in relation to the oocyte retrieval
period. In this case, D0 representing the day of retrieval and
assessment of the oocyte, D1 the fertilization day and zygote
observation, D2 and D3 for the cleavage and embryo stage evaluation, D4
to D6 for the day of assessing morulae and blastocyst (Alpha Scientists in Reproductive & ESHRE Special Interest Group of Embryology, 2011).

Figure 1. Questionnaire given to Brazilian embryologists.
d. Media profiles
The media reported in the questionnaire were the following: Cleavage
and Blastocyst from Cook Culture™, GV blast from Ingámed™, Continuous
Single Culture™ (CSCM™) and Continuous Single Culture-Complete™
(CSCM-C™) from Irvine Scientific™ and Global ™ from LifeGlobal. Since
some embryologists answered just Vitrolife without specifying the media
type, there are compound specifications [Figure 2] for those four media from this brand available in Brazil, which are G-1™, G-1™ PLUS, G-2™ and G-2™ PLUS.
The embryo cultivation media were separated in terms of brands and
broken down according to their chemical characteristics, which is
presented on table 2. Essential and nonessential amino acids are
present in Blastocyst and Cleavage Medium from Cook™, CSCM™ and CSCM-C™
from Irvine Scientific, Global™ from Life Global and G2™ and G2™ Plus
from Vitrolife. The culture media G1™ and G1™ plus from Vitrolife bear
only nonessential amino acids. Only three media have dipeptides in
their composition, they are: CSCM™ and CSCM-C™ from Irvine Scientific
and Global™ from LifeGlobal. The antibiotic is the same for all culture
media: gentamicin. Antioxidants are present just in the Cleavage Medium
from Cook™, in both media from Irvine Scientific and in Global™ from
LifeGlobal. All of the media reported in the questionnaire have sodium
bicarbonate as a buffer. As far as energy substrates are concerned,
only Vitrolife had just one type (hyaluronan); in short, more types of
energy supplies can be found in other media (e.g. D-glucose, glucose,
Sodium lactate, Sodium L-lactate, Sodium Pyruvate). The pH is indicated
by phenol red only in the media from Irvine Scientific and LifeGlobal.
Human serum albumin (HSA) is already supplemented in media from Cook™,
CSCM-C™ from Irvine Scientific and in G1™ plus and G2™ plus from
Vitrolife. It is important to remember that products containing human
originated protein in their compositions have the potential presence of
contaminants sourced from such obscure nature protein (Morbeck et al.,
2014). The other brands have formulations with or without protein. Salt
and ions are present in most of them, but in case of Vitrolife this
information was not clear enough to be along with the others.
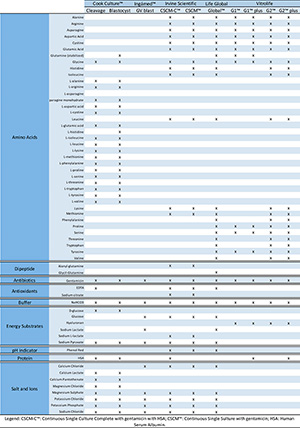
Figure 2. Que Chemical characteristics of the embryo cultivation media
most used in Brazil, by brand, medium type and chemical class.
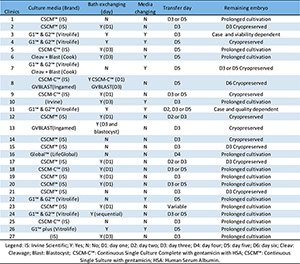
Figure 3. Summary of the answers to a 5-items questionnaire given to
embryologists from Brazilian ART clinics about embryo culture media
used.
RESULTS
After data collection, figure 3
was created to better show the content of each answer. In the case of
utilized media, the most used was CSCM™, represented by 10 clinics
(37.04%) which responded the questionnaire. The second most used was
media from Vitrolife, represented by 7 clinics (25.93%). Thirdly, 5
clinics (18.52%) reported the use of CSCM-C™. In fourth came 2 clinics
(7.40%) that were using GV Blast and other 2 clinics (7.40%) were using
media from Cook. Lastly, only 1 clinic was using Global™ media.
About the second question, 19 clinics (70.37%) answered they were used
to bath exchange and 8 clinics (29.63%) were not. To some answers, the
respondents added the day in which the bath was changed; D1 happened in
9 clinics (33%) and D3 in 6 clinics (22%).
In the third question, 70.37% of the responses were negative, compared to 29.63% that were positive for changing media type.
The question about the day of transferring, 37.04% of the clinics
transfer on D3; 25.93% on D5; 18.52% on D3 or D5; 7.40% on D2 or D3.
The remaining 11.10% were equally distributed in three different
patterns: transfers occurring on D2, D3 or D5; D4; and variable
depending on the case.
In the last question, the majority of clinics, represented by 51.85%,
keep embryos in prolonged cultivation; others 18.52% answered that the
embryos were cryopreserved, but did not specify the date; 14.81% of the
clinics used to freeze the embryos on D3; 7.40% of the interviewees
answered it would depend on each case. Lastly, 1 clinic (3.70%)
cryopreserves embryos on D6 and another clinic (3.70%) cryopreserves on
D3 or D5.
DISCUSSION
The difference in
the number of clinics and egg transfers in Europe, USA and Brazil can
be explained by three main topics. First, those numbers are related to
the quantity of countries included, the country level of development
and characteristics such as environment and socioeconomic demographics.
In second, the age of the population and cultural criteria influence
family planning. Lastly and most relevant to IVF procedures, the
earlier the embryo is transferred the more number of transfers can be
carried out. In short, it is not just about being a developed or
developing country that counts for the number of ART procedures.
An important discrepancy seen between the results from Brazilian
clinics and the literature is about the embryo culture media. Data,
described before in this paper, showed that the media is made in
laboratory; however, none of the clinics answered the questionnaire
with a single media done in their own laboratories. In short, although
it has been described as a possible media handling, especially in Latin
America, in Brazil it does not appear to be common.
In conclusion, embryonic development and successful IVF treatment
relies on embryo culture medium. This is a fact that can be proved by
numerous literature papers in addition to the high cost of media from
diverse companies. Even though the embryo media importance is known,
there is a lack of studies comparing media and enough evidence to
support or refute one specific medium.
REFERENCES
ANVISA - Agência Nacional de Vigilância Sanitária [Internet]. 8º Relatório do Sistema Nacional de Produção de Embriões - 2015. Available at: http://portal.anvisa.gov.br/wps/wcm/connect/9cddb8004840da35a438a5bdc15bfe28/sisembrio8.pdf?MOD=AJPERES. Accessed: 02/02/2016.
Alpha
Scientists In Reproductive M, ESHRE Special Interest Group of
Embryology. The Istanbul consensus workshop on embryo assessment:
proceedings of an expert meeting. Hum Reprod. 2011; 26: 1270-83.
Medline Crossref
Centers for Disease Control and Prevention. Assisted Reproductive Technology. Assisted Reproductive Technology National Summary Report 2013. Atlanta (GA): US Dept of Health and Human Services; 2015. Availabe at: http://www.cdc.gov/art/pdf/2013-report/art_2013_national_summary_report.pdf. Accessed: 02/02/2016.
Chronopoulou E, Harper JC. IVF culture media: past, present and future. Hum Reprod Update. 2015; 21:39-55.
Medline Crossref
ESHRE - European Society of Human Reproduction and Embryology [Internet]. ART fact sheet – 2014. Available at: https://www.eshre.eu/guidelines-and-legal/art-fact-sheet.aspx. Accessed: 02/02/2016.
European
IVF-Monitoring Consortium (EIM); European Society of Human Reproduction
and Embryology (ESHRE), Kupka MS, D’Hooghe T, Ferraretti AP, de Mouzon
J, Erb K, Castilla JA, Calhaz-Jorge C, De Geyter Ch, Goossens V.
Assisted reproductive technology in Europe, 2011: results generated
from European registers by ESHREdagger. Hum Reprod. 2016;31: 233-48.
Medline Crossref
Kleijkers
SH, Eijssen LM, Coonen E, Derhaag JG, Mantikou E, Jonker MJ,
Mastenbroek S, Repping S, Evers JL, Dumoulin JC, van Montfoort AP.
Differences in gene expression profiles between human preimplantation
embryos cultured in two different IVF culture media. Hum Reprod.
2015;30:2303-11.
Medline Crossref
Marianowski
P, Szymusik I, Grzechocinska B, Cyganek A. The comparison of two
different embryo culture methods in the course of in vitro
fertilization program. Folia Histochem Cytobiol. 2007; 45:S115-7.
Medline
REDLARA -Red Latinamericana de Reproducción Asistida [Internet]. Available from: http://www.redlara.com/aa_ingles/default.asp. Accessed: 02/02/2016.
REDLARA - Red Latinamericana de Reproducción Asistida. Manual de Procedimentos – Laboratório de Reprodução Assistida 2006. Available from: http://www.redlara.com/images/arq/livreto_port_01_2007.pdf. Accessed: 02/02/2016.
Sicignano
N, Beydoun HA, Russell H, Jones HJr, Oehninger S. A descriptive study
of asthma in young adults conceived by IVF. Reprod Biomed Online.
2010;21:812-8.
Medline Crossref
Youssef
MM, Mantikou E, Van Wely M, Van Der Veen F, Al-Inany HG, Repping S,
Mastenbroek S. Culture media for human pre-implantation embryos in
assisted reproductive technology cycles. Cochrane Database Syst Rev.
2015;11:CD007876.
Medline Crossref