JBRA Assist. Reprod. 2017;21(3):183-187
ORIGINAL ARTICLE
doi: 10.5935/1518-0557.20170036
Comparison of two different dosage of GnRH agonist as ovulation trigger in oocyte donors: a randomized controled trial
1Assisted Reproductive Medicine Unit. Ginemed Clinics, Sevilla-Spain
CONFLICT OF INTERESTS
No conflict of interest has been declared.
ABSTRACT
Objective: To compare the results obtained with two different GnRH agonist dosages: 0.3mg versus 0.4mg to trigger ovulation in oocyte donor cycles.
Methods: Experimental controlled randomized trial including 40 patients from a private practice center. The patients were randomized into two groups. Group A received a single dose of Triptorelin 0.3mg (Decapeptyl®) 36hours before pick-up. Group B patients received Triptorelin 0.4mg (Decapeptyl®) before pick-up to final oocyte maturation. We evaluated the total number of oocytes collected, the number of mature oocytes and total days of ovarian stimulation.
Results: The average of total collected oocytes were 16 (Group A) versus 15 (Group B), and the mean number of mature oocytes were 13 versus 12 respectively. The only variable showing a difference was the percentage of mature oocytes, which was greater in Group A, resulting in 84.6%, in contrast with those treated with 0.4mg of Triptorelin (78.6%), although these differences were not statistical significant (p=0.35). Days of stimulation did not differ between groups. No cases of empty follicle syndrome were reported.
Conclusions: We found that an increase from 0.3 to 0.4mg of triptorelin in an oocyte donation program might not improve outcomes. Nevertheless, more studies might be necessary, not only in oocyte donors but in sterile women as well, to evaluate how GnRH agonist dosage could affect the results among other factors.
Keywords: oocytes, oocyte Donation, ovulation, Gn-RH
INTRODUCTION
Classically, assisted reproductive treatments (ART) have employed hCG as a substitute for endogenous LH surge, to induce oocyte maturation. hCG offers structural similarities to LH, acting on the same receptors. Its main difference lies in the longer half-life of hCG. The increased and lasting LH surge may cause an ovarian hyperstimulation syndrome (OHSS) (Damewood et al. 1989; Whelan & Vlahos, 2000). OHSS is one of the most feared complications of ART, causing high comorbidity among patients submitted to this type of treatment (Golan et al., 1989). Because the GnRH antagonist does not desensitize hypophysis receptors, as they were introduced, a new window of possibilities to trigger LH surge using GnRH agonist was opened (Olivennes et al., 1996; Tarlatzis et al., 2006; Griesinger et al., 2006). The GnRH agonist bolus causes an initial FSH and LH discharge from the hypophysis, a "flare up" effect, resembling the one caused by a natural cycle, and enough to reduce oocyte maturation, thus the risk of OHSS (Hoff et al., 1983; Pundir et al., 2012; Bodri et al., 2010; Engmann et al., 2008). Itskovitz et al. (2000) were the first to suggest GnRH agonist as an alternative to prevent OHSS. Today, a GnRH agonist bolus is used as the standard method to trigger final maturation in young patients, or in women with an increased ovarian reserve due to its relevant safety profile. Although its usage has been standardized lately, there still are unsolved questions about its usage.
One of the main disadvantages of employing GnRH agonists, is the decreased pregnancy rate reported after fresh embryo transference. This effect has been attributed to luteal phase deficiency, which affects endometrial quality (Youssef et al., 2011; Babayof et al., 2006). Several trials have demonstrated similar pregnancy rates using different luteal phase support combinations; however, none have achieved enough evidence - reason why the freeze-all policy and delayed embryo transfer is the most commonly used method after GnRH agonist bolus (Youssef et al., 2014; Radesic & Tremellen, 2011; Papanikolaou et al., 2011; Humaidan et al., 2011).
Besides, another difficulty concerning the use of GnRH agonist is the standard dosage to achieve a sufficient LH surge for follicular maturation. Suboptimal results have been described after GnRH bolus, due to a deficient LH surge (Castillo et al., 2012). Numerous dosage protocols have been proposed to trigger follicular maturation; from as low as 0.1mg to as high as 0.4mg, without having clear evidence of neither one's benefit (Buckett et al., 1998; Emperaire et al., 2004).
The goal of our trial was to evaluate the number of collected mature oocytes (MII) after controlled ovarian stimulation, using a fixed gonadotropin (rFSH) protocol and multiple GnRH agonist doses to trigger final maturation.
MATERIAL AND METHODS
Experimental controlled randomized trial including 40 women belonging to a single medical care center, carried out from January to March 2016. All patients signed an informed consent. The trial fulfilled Helsinki's declaration, good clinical practice and decree law 9/2014 to regulate oocyte donation.
Studied population
Our study included only oocyte donors in which oocyte maturation was accomplished using GnRH agonist. The inclusion criteria were: age ranging 18-30 years, BMI<30. No history of diseases contraindicating the treatment, recent normal gynecological control, ≥16 antral follicles and satisfying results in previous cycles (>8 mature oocytes or achieved pregnancy).
We also aimed at analyzing secondary variables, such as the total number of collected oocytes, mature oocyte percentage and total days of ovarian stimulation.
Procedure
The patients were randomized into two groups. Randomization was performed through randomization tables, for a total of 40 patients included. When oocyte donors came to the clinic to initiate the cycle, the medical staff on duty that day used the table for randomization and assigned the patient to one of the two groups.
Group A received a single dose of Triptorelin 0.3mg (Decapeptyl®) 36hours before pick-up. Group B received Triptorelin 0.4mg (Decapeptyl®) 36hours before pick-up for final maturation.
Statistical analysis
Qualitative variables were represented as absolute frequencies and percentages, and were analyzed in a descriptive way. The quantitative variables were resumed in average (standard deviation) or median (p25; p75), according to their asymmetry. Normality was assessed by the Shapiro-Wilk's test and homogeneity by the Levenne's test.
Losses were analyzed in each group using the Fisher's exact test. Age and BMI were evaluated by the Student T-test, acquiring a 95% interval of confidence for the differences. Remaining variables were analyzed using the Mann-Whitney test. Statistical significance was set at 5% (p<0.05). the IBM SPSS19.0 software was used for complete analysis.
RESULTS
A total of 40 patients were included in the trial, all were previously randomized into two groups: Group A (n=20) and Group B (n=20). 4 patients did not complete the study. Two patients in Group A rejected donation and one was cancelled because of unsatisfactory response to stimulation. In Group B, one patient was cancelled for insufficient response. Losses were similar in both groups (p=0.605). The 36 remaining patients were 34 years old in average and had BMI of 22.9. The mean number of collected oocytes was 15.5 (12; 30.5). Eighty-six point-one percent of the women had 12 days of controlled ovarian stimulation.
The group assessment did not confirm significant age differences. Group A and B showed similar mean values (23.9 vs. 24.2 years old). A minor difference in BMI was found between the groups: Group B had 2.48 units more than Group A (Table 1).
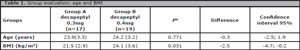
Table 1. Group evaluation: age and BMI
The average of collected oocytes were 13 vs. 12, and the total numbers were 16 vs. 15. The single variable showing a difference was the percentage of mature oocytes which was greater in the 0.3mg group (A), resulting in 84.6(71.5; 88.3), in contrast with those treated with 0.4 mg: 78.6 (65.6; 86.6), although this difference was not statistically significant. Days of stimulation did not differ between groups (Table 2). No cases of Empty Follicle Syndrome (EFS) were reported.
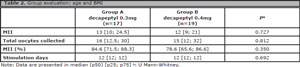
Table 2. Group evaluation: age and BMI
DISCUSSION
Our study, which compared the usage of triptorelin 0.3mg and 0.4mg in oocyte donors, did not show any difference in the number of collected oocytes, metaphase II, or days of stimulation.
GnRH-agonist-induced FSH and LH surges resembles the natural cycle in a better way than hCG bolus, although the LH surge is reduced and shorter when compared to the natural cycle. This situation induces luteal phase insufficiency, luteal corps' injury and decreased LH levels (Yding Anderesen et al., 1999; Beckers et al., 2003; Humaidan et al., 2012). Nevertheless, the advantage brought about by the GnRH agonist is the FSH surge, which stimulates the development of LH receptors in the granulosa cells, optimizing luteal corps' function (Zelinski-Wooten et al., 1995; Humaidan et al., 2009). Moreover, GnRH agonist endorses oocyte maturation, reinitiating meiosis and cumulus' expansion, and reporting high mature oocyte rates (Andersen et al., 2006; Humaidan et al., 2011).
Several GnRH agonists, such as buserelin, leuprolide acetate and triptorelin, have proven useful for oocyte maturation (Bodri et al., 2009; Engmann et al., 2008; Humaidan et al., 2005). Parneix et al. (2001) evaluated thirteen different protocols for GnRH agonist bolus, and they concluded that none showed differences triggering maturation.
The dosages used vary from 0.1mg to 0.5mg. Trying to establish the most effective dosage, Vuong et al. (2016) published a trial including oocyte donors and comparing different triptorelin doses (0.2mg, 0.3mg and 0.4mg). It included 165 patients randomized into three groups, 55 women in each. No differences were found among the main variables: mature oocytes and good quality embryos. Though, statistical differences were found on LH levels, higher in the 0.4mg group compared to 0.2 and 0.3mg groups. Progesterone levels in day 6 were also found to be higher in the 0.4mg group. It remains to elucidate if the lower LH levels could cause clinical impairment as an increase in the number of empty follicle syndrome (EFS) cases (Stevenson & Lashen, 2008; Beck-Fruchter et al., 2012).
Although these cases have been like a-GnRH and hCG cases, the mechanism causing it might be different (Castillo et al., 2010). Different theories have been proposed, among them: ovarian aging, insufficient dosage and genetic receptor changes (Toledo et al., 1996; Aktas et al., 2005). Blazquez et al. (2014) published a retrospective study including 12,483 oocyte donation cycles. There were 74 cases of EFS (0.59%) described from 2006 to 2013. hCG was used to trigger maturation until 2009; latter, triptorelin at 0.2 or 0.3mg were used. Although more cases were reported in the 0.2mg group, they were not statistically significant. The Empty Follicle Syndrome incidence has not been shown to be higher with the use of GnRH, nor has it been possible to demonstrate a higher incidence with lower doses, because of its low prevalence and few studies. There were no cases of this syndrome reported in our study.
CONCLUSIONS
Thus, we believe that an increase from 0.3 to 0.4 mg in an oocyte donation program might not provide any outcome benefits. Nevertheless, more studies might be necessary, not only among oocyte donors, but also in sterile women, to evaluate how GnRH agonist dosage might differ according to other factors.
DISCLAIMERS
The opinions expressed in this paper are those of the authors and does not reflect that of to the position of the assigned institution.
SOURCE OF SUPPORT
The drugs and other resources used in the patients included in this study, where kindly provided by GINEMEDs Clinics, as part of the normal function and management of the oocyte donors department. We did receive financial funds from external entities.
REFERENCES
Aktas M, Beckers NG, van Inzen WG, Verhoeff A, de Jong D. Oocytes in the empty follicle: a controversial syndrome. Fertil Steril. 2005;84:1643-8.
Medline Crossref
Andersen CY, Humaidan P, Ejdrup HB, Bungum L, Grøndahl ML, Westergaard LG. Hormonal characteristics of follicular fluid from women receiving either GnRH agonist or hCG for ovulation induction. Hum Reprod. 2006;21:2126-30.
Medline Crossref
Babayof R, Margalioth EJ, Huleihel M, Amash A, Zylber-Haran E, Gal M, Brooks B, Mimoni T, Eldar-Geva T. Serum inhibin A, VEGF and TNFalpha levels after triggering oocyte maturation with GnRH agonist compared with HCG in women with polycystic ovaries undergoing IVF treatment: a prospective randomized trial. Hum Reprod. 2006;21:1260-5.
Medline Crossref
Beck-Fruchter R, Weiss A, Lavee M, Geslevich Y, Shalev E. Empty follicle syndrome: successful treatment in a recurrent case and review of the literature. Hum Reprod. 2012;27:1357-67.
Medline Crossref
Beckers NG, Macklon NS, Eijkemans MJ, Ludwig M, Felberbaum RE, Diedrich K, Bustion S, Loumaye E, Fauser BC. Nonsupplemented luteal phase characteristics after the administration of recombinant human chorionic gonadotropin, recombinant luteinizing hormone, or gonadotropin-releasing hormone (GnRH) agonist to induce final oocyte maturation in in vitro fertilization patients after ovarian stimulation with recombinant follicle-stimulating hormone and GnRH antagonist cotreatment. J Clin Endocrinol Metab. 2003;88:4186-92.
Medline Crossref
Blazquez A, Guillén JJ, Colomé C, Coll O, Vassena R, Vernaeve V. Empty follicle syndrome prevalence and management in oocyte donors. Hum Reprod. 2014;29:2221-7.
Medline Crossref
Bodri D, Guillén JJ, Galindo A, Mataró D, Pujol A, Coll O. Triggering with human chorionic gonadotropin or a gonadotropin-releasing hormone agonist in gonadotropin-releasing hormone antagonist-treated oocyte donor cycles: findings of a large retrospective cohort study. Fertil Steril. 2009;91:365-71.
Medline Crossref
Bodri D, Guillén JJ, Trullenque M, Schwenn K, Esteve C, Coll O. Early ovarian hyperstimulation syndrome is completely prevented by gonadotropin releasing-hormone agonist triggering in high-risk oocyte donor cycles: a prospective, luteal-phase follow-up study. Fertil Steril. 2010;93:2418-20.
Medline Crossref
Buckett WM, Bentick B, Shaw RW. Induction of the endogenous gonadotrophin surge for oocyte maturation with intra-nasal gonadotrophin-releasing hormone analogue (buserelin): effective minimal dose. Hum Reprod. 1998;13:811-4.
Medline Crossref
Castillo JC, Dolz M, Bienvenido E, Abad L, Casañ EM, Bonilla-Musoles F. Cycles triggered with GnRH agonist: exploring low-dose HCG for luteal support. Reprod Biomed Online. 2010;20:175-81.
Medline Crossref
Castillo JC, Garcia-Velasco J, Humaidan P. Empty follicle syndrome after GnRHa triggering versus hCG triggering in COS. J Assist Reprod Genet. 2012;29:249-53.
Medline Crossref
Damewood MD, Shen W, Zacur HA, Schlaff WD, Rock JA, Wallach EE. Disappearance of exogenously administered human chorionic gonadotropin. Fertil Steril. 1989;52:398-400.
Medline Crossref
Emperaire JC, Parneix I, Ruffie A. Luteal phase defects following agonist-triggered ovulation: a patient-dependent response. Reprod Biomed Online. 2004;9:22-7.
Medline Crossref
Engmann L, DiLuigi A, Schmidt D, Nulsen J, Maier D, Benadiva C. The use of gonadotropin-releasing hormone (GnRH) agonist to induce oocyte maturation after cotreatment with GnRH antagonist in high-risk patients undergoing in vitro fertilization prevents the risk of ovarian hyperstimulation syndrome: a prospective randomized controlled study. Fertil Steril. 2008;89:84-91.
Medline Crossref
Golan A, Ron-el R, Herman A, Soffer Y, Weinraub Z, Caspi E. Ovarian hyperstimulation syndrome: an update review. Obstet Gynecol Surv. 1989;44:430-40.
Medline Crossref
Griesinger G, Diedrich K, Devroey P, Kolibianakis EM. GnRH agonist for triggering final oocyte maturation in the GnRH antagonist ovarian hyperstimulation protocol: a systematic review and meta-analysis. Human Reprod Update. 2006;12:159-68.
Medline Crossref
Hoff JD, Quigley ME, Yen SS. Hormonal dynamics at midcycle: a reevaluation. J Clin Endocrinol Metab. 1983;57:792-6.
Medline Crossref
Humaidan P, Bredkjaer HE, Bungum L, Bungum M, Grøndahl ML, Westergaard L, Andersen CY. GnRH agonist (buserelin) or hCG for ovulation induction in GnRH antagonist IVF/ICSI cycles: a prospective randomized study. Hum Reprod. 2005;20:1213-20.
Medline Crossref
Humaidan P, Papanikolaou EG, Tarlatzis BC. GnRHa to trigger final oocyte maturation: a time to reconsider. Hum Reprod. 2009;24:2389-94.
Medline Crossref
Humaidan P, Kol S, Papanikolaou EG; Copenhagen GnRH Agonist Triggering Workshop Group. GnRH agonist for triggering of final oocyte maturation: time for a change of practice? Hum Reprod Update. 2011a;17:510-24.
Medline Crossref
Humaidan P, Westergaard LG, Mikkelsen AL, Fukuda M, Yding Andersen C. Levels of the epidermal growth factor-like peptide amphiregulin in follicular fluid reflect the mode of triggering ovulation: a comparison between gonadotrophin-releasing hormone agonist and urinary human chorionic gonadotrophin. Fertil Steril. 2011b;95:2034-8.
Medline Crossref
Humaidan P, Papanikolaou EG, Kyrou D, Alsbjerg B, Polyzos NP, Devroey P, Fatemi HM. The luteal phase after GnRH-agonist triggering of ovulation: present and future perspectives. Reprod Biomed Online. 2012;24:134-41.
Medline Crossref
Itskovitz-Eldor J, Kol S, Mannaerts B. Use of a single bolus of GnRH agonist triptorelin to trigger ovulation after GnRH antagonist ganirelix treatment in women undergoing ovarian stimulation for assisted reproduction, with special reference to the prevention of ovarian hyperstimulation syndrome: preliminary report: short communication.Hum Reprod. 2000;15:1965-8.
Crossref
Olivennes F, Fanchin R, Bouchard P, Taieb J, Frydman R. Triggering of ovulation by a gonadotropin-releasing hormone (GnRH) agonist in patients pretreated with a GnRH antagonist. Fertil Steril. 1996;66:151-3.
Medline Crossref
Papanikolaou EG, Verpoest W, Fatemi H, Tarlatzis B, Devroey P, Tournaye H. A novel method of luteal supplementation with recombinant luteinizing hormone when a gonadotropin-releasing hormone agonist is used instead of human chorionic gonadotropin for ovulation triggering: a randomized prospective proof of concept study. Fertil Steril. 2011;95:1174-7.
Medline Crossref
Parneix I, Emperaire JC, Ruffie A, Parneix P. Comparison of different protocols of ovulation induction, by GnRH agonists and chorionic gonadotropin. Gynecol Obstet Fertil. 2001;29:100-5.
Medline Crossref
Pundir J, Sunkara SK, El-Toukhy T, Khalaf Y. Meta-analysis of GnRH antagonist protocols: do they reduce the risk of OHSS in PCOS? Reprod Biomed Online. 2012;24:6-22.
Medline Crossref
Radesic B, Tremellen K. Oocyte maturation employing a GnRH agonist in combination with low-dose hCG luteal rescue minimizes the severity of ovarian hyperstimulation syndrome while maintaining excellent pregnancy rates. Hum Reprod. 2011;26:3437-42.
Medline Crossref
Stevenson TL, Lashen H. Empty follicle syndrome: the reality of a controversial syndrome, a systematic review. Fertil Steril. 2008;90:691-8.
Medline Crossref
Tarlatzis BC, Fauser BC, Kolibianakis EM, Diedrich K, Rombauts L, Devroey P. GnRH antagonists in ovarian stimulation for IVF. Hum Reprod Update. 2006;12:333-40.
Medline Crossref
Toledo SP, Brunner HG, Kraaij R, Post M, Dahia PL, Hayashida CY, Kremer H Themmen AP. An inactivating mutation of the luteinizing hormone receptor causes amenorrhea in a 46,XX female. J Clin Endocrinol Metab. 1996;81:3850-4.
Medline Crossref
Vuong TN, Ho MT, Ha TD, Phung HT, Huynh GB, Humaidan P. Gonadotropin-releasing hormone agonist trigger in oocyte donors co-treated with a gonadotropin-releasing hormone antagonist: a dose-finding study. Fertil Steril. 2016;105:356-63.
Medline Crossref
Whelan JG 3rd, Vlahos NF. The ovarian hyperstimulation syndrome. Fertil Steril. 2000;73:883-96.
Medline Crossref
Yding Andersen C, Leonardsen L, Ulloa-Aguirre A, Barrios-De-Tomasi J, Moore L, Byskov AG. FSH-induced resumption of meiosis in mouse oocytes: effect of different isoforms. Mol Hum Reprod. 1999;5:726-31.
Medline Crossref
Youssef MA, Van der Veen F, Al Inany HG, Griesinger G, Mochtar MH, Aboulfoutouh I, Khattab SM, van Wely M. Gonadotropin-releasing hormone agonist versus HCG for oocyte triggering in antagonist assisted reproductive technology cycles. Cochrane Database Syst Rev. 2011;(1):CD008046.
Medline Crossref
Youssef MA, Van der Veen F, Al-Inany HG, Griesinger G, Mochtar MH, van Wely M. Gonadotropin-releasing hormone agonist versus HCG for oocyte triggering in antagonist assisted reproductive technology cycles. Cochrane Database Syst Rev. 2014;(10):CD008046.
Medline Crossref
Zelinski-Wooten MB, Hutchison JS, Hess DL, Wolf DP, Stouffer RL. Follicle stimulating hormone alone supports follicle growth and oocyte development in gonadotrophin-releasing hormone antagonist-treated monkeys.Hum Reprod. 1995;10:1658-66.
Medline Crossref