JBRA Assist. Reprod. 2017;21(3):260-272
REVIEW
doi: 10.5935/1518-0557.20170048
Fresh embryos versus freeze-all embryos - transfer strategies: Nuances of a meta-analysis
1Center for Human Reproduction Prof. Franco Jr., Ribeirão Preto, Brazil
2Paulista Center for Diagnosis, Research and Training, Ribeirão Preto, SP, Brazil
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
This study was presented at the 33th annual meeting of ESHRE 2017, in Geneva, Switzerland
ABSTRACT
Objective The present meta-analysis aimed to evaluate whether the freeze-all strategy (Freeze/All-ET) could bring about improvements in the clinical assisted reproductive technique (ART) outcomes when compared with the fresh embryo transfer strategy (Fresh-ET) in patients undergoing an ART cycle in accordance with the mean number of oocytes collected.
Methods A systematic review based on electronic searches in databases (PubMed, EMBASE, Web of Science, SCOPUS, and Cochrane Central Register of Controlled Trials) was carried out to identify randomized controlled trails (RCTs) comparing ART outcomes between fresh-embryo transfers versus elective frozen-embryo transfers up to February of 2017. Four reviewers independently evaluated abstracts, validity assessment and data extraction. Odds Ratio (OR) values with a 95% confidence interval (CI), and heterogeneity were evaluated.
Results Five RCTs were included as targets for data extraction and meta-analysis purposes. The results of this meta-analysis were divided into two parts (Freeze/All-ET versus Fresh-ET): Part I- All trials in which the mean number of collected oocytes was >12 and <21 for ongoing pregnancy rate (OR=1.24; 95%CI=1.06-1.44), clinical pregnancy rate (OR=1.19; 95%CI=0.98-1.43), live birth rate (OR= 1.39; 95%CI=0.99-1.95), and miscarriage rate (OR=0.68; 95%CI=0.46-1.00); Part II- Three studies where the mean number of oocytes retrieved was >12 and <15 for ongoing pregnancy rate (OR=1.17; 95%CI=1.00-1.38), clinical pregnancy rate (OR=1.34; 95%CI=0.79-2.28), live birth rate (OR= 1.24; 95%CI=1.00-1.55), and miscarriage rate (RR=0.68; 95%CI=0.46-1.02).
Conclusions The freeze-all strategy could be favorable when high numbers of oocytes are collected, signaling an association between higher ovarian stimulation and consequent impairment of endometrial receptivity. However, when the mean number of oocytes collected is <15, the freeze-all strategy does not appear to be advantageous.
Keywords: Freeze-all embryos, fresh-embryo transfer, ovarian stimulation, endometrial receptivity, IVF/ICSI, meta-analysis
INTRODUCTION
The Freeze-all strategy (Freeze/All-ET), which consists of the cryopreservation of all embryos from an assisted reproductive technique (ART) cycle, and delayed embryo transfer in a natural cycle or a programmed hormone replacement cycle to prepare the endometrium, which is considered the preferred way to avoid potential deleterious effects of controlled ovarian stimulation (COS) during fresh-embryo transfer (Fresh-ET) on endometrium receptivity, and consequently on embryonic implantation (Silverberg et al., 1994; Shapiro et al., 2008). COS is associated with negative effects on endometrial receptivity during ART cycles, probably due to high levels of estrogen (E) and progesterone (P) during the follicular phase compared to natural cycles (Kolibianakis et al., 2002; Bosch et al., 2003; Venetis et al., 2013; Huang et al., 2015). Because of subtle elevations of P during COS, there could be a consequent asynchrony between the endometrium and the transferred embryos; probably the endometrial development should be at an advanced stage at the moment of embryonic implantation (Nikas, 1999; Wong et al., 2014). Therefore, it's known that the best results in ART, considering pregnancy rates, are found in oocyte donation cycles and cycles using frozen-thawed embryos transfer (FET) (Murata et al., 2005; Richter et al., 2006; Shapiro et al., 2009; Kansal Kalra et al., 2011). A plausible explanation for this is the fact that the endometrium is artificially primed, without COS and supraphysiological hormonal levels at the time of the embryo transfer (Melo et al., 2006; Venetis et al., 2013). In addition, it has been reported that patients with high ovarian reserve, e.g. high-risk of ovarian hyperstimulation syndrome (OHSS), and polycystic ovarian syndrome (PCOS) patients, could benefit from the Freeze/All-ET (Griesinger et al., 2007; Griesinger et al., 2011).
The aim of the present systematic review and meta-analysis is to evaluate whether Freeze/All-ET could bring about improvements in the clinical ART outcomes when compared with Fresh-ET in patients undergoing the ART cycle, in accordance with the mean number of oocytes collected.
MATERIALS AND METHODS
Identification of studies
We ran a systematic review based on electronic searches in the following databases (PubMed, EMBASE, Web of Science, SCOPUS, and Cochrane Central Register of Controlled Trials), up to February of 2017, to identify randomized controlled trials (RCTs) comparing ART outcomes of Freeze/All-ET versus Fresh-ET. The search was restricted to papers published in English. The following medical subject headings and text words were used: "IVF", "ICSI", "freeze-all", "frozen-thawed embryos", "frozen-embryo transfer", "fresh-embryo transfer", "poor-responder", "normal-responder", "high-responder", "clinical outcomes", "oocytes collected", and "randomized study". The main inclusion criterion was a randomized controlled trial (RCT).
Criteria for including studies in this meta-analysis
All available published and ongoing randomized controlled trials comparing clinical outcomes between patients undergoing IVF/ICSI cycles with Freeze/All-ET or Fresh-ET were included. All trials provided data on IVF cycles, including number of oocytes retrieved.
Outcome measures
The primary outcome measure for this meta-analysis was the ongoing pregnancy rates (per woman, randomized). Secondary outcomes included clinical pregnancy rates (per patient randomized) and miscarriage rates (from clinical pregnancy). Clinical pregnancy was defined as the presence of a gestational sac in the uterine cavity (with or without a heartbeat) at 6/7 gestation week, detected by ultrasonography. Ongoing pregnancy was defined as the presence of a fetus with heart motion at 10 to 12 weeks of gestation. Miscarriage was considered any pregnancy - clinical pregnancy - that did not achieve ongoing pregnancy status. In addition, live birth rates defined as the delivery of a live-born infant after 25 weeks of gestation was included as secondary outcomes.
Validity assessment and data extraction
Each trial was assessed independently by four reviewers (FCD, JBAO, RLRB and JGF), and ranked for its methodological rigor and its potential for the introduction of biases. Originally reported characteristics, including a method for randomization, the presence of a power calculation, the unit of analysis used, and the presence or absence of examiner blinding were analyzed. Missing data were obtained from the authors.
Statistical analysis
Five RCTs were included as targets for data extraction and meta-analysis. The data was combined for meta-analysis using the Stats-Direct statistical software. Dichotomous data was expressed as Odds Ratio (OR) with a 95% confidence interval (CI). The measure of heterogeneity was evaluated using Cochran's Q and I2. The heterogeneity was considered high when I2≥50%. The study data was combined using a fixed-effects model when the heterogeneity among the trials was considered low or statistically insignificant (I 2 was <50%). However, the random-effects model was employed when the heterogeneity was considered substantial (I2≥50%), and when I2 was not applicable (NA). P-values<0.05 were considered statistically significant. The present meta-analysis was reported following the Preferred Reporting Item for Systematic Reviews and Meta-analyses (PRISMA) statement (S1 File).
RESULTS
Study selection and characteristics
Among the 72 potentially relevant studies found, a total of five trials fulfilled the inclusion criteria (Shapiro et al., 2011a; Shapiro et al., 2011b; Chen et al., 2016; Vuong et al., 2016; Coates et al., 2017). A flow diagram of the selection process is depicted in Figure 1. From the studies included, 2,728 patients were enrolled; 1,358 in the Freeze/All-ET group and 1,370 in the Fresh-ET group. The sample sizes of the included trials ranged between 60 and 762 women. The main characteristics and description of the five RCTs included in this meta-analysis are shown on Table 1 and the literature-exclusion procedures are available in Figure 1.
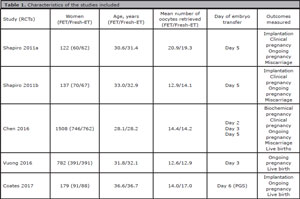
Table 1. Characteristics of the studies included
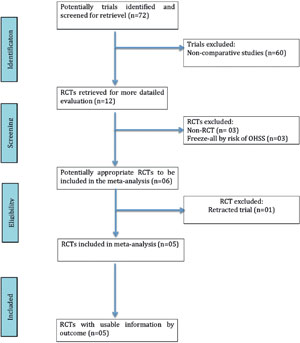
Figure 1. QUOROM statement flow diagram illustrating the selection of trials included in this meta-analysis.
Systematic Review
Shapiro et al., 2011a (High-responder): A prospective randomized trial was performed to assess potential effects of COS on endometrial receptivity. It was published, as correspondence, thus complete data on methods were not evaluated ("not-randomized", "not-blind", "no-power calculation" descriptions). Clinical pregnancy rates per transfers in Freeze/All-ET cycles and Fresh-ET were compared. The inclusion criteria were patients undergoing their first IVF cycle, day 3 FSH cycle <10IU/L, and >15 antral follicle-count. This study involved 131 patients, and 122 were randomized (62 to the fresh group and 60 to the cryopreservation group). The two groups were similar in age, antral follicle count, days of stimulation (10.4 versus 10.6), mean number of oocytes retrieved for Freeze/All-ET group (20.9±8.2) and Fresh-ET group (19.3±8.6), etc. The ongoing pregnancy rates per retrieval were 63.3% (38/60) in the Freeze/All-ET group and 54.8% (34/62) in the Fresh-ET group (p=0.36). Regression logistics was performed to check for potential differences in clinical outcomes while controlling for embryo quality. They found that a greater likelihood of clinical pregnancy was associated with the Freeze/All-ET group (p=0.0037).
Shapiro et al., 2011b (Normo-responder): In this prospective randomized study of 137 patients undergoing their first IVF cycle in which they had 67 and 70 oocytes retrieved in the Fresh-ET and Freeze/All-ET groups, respectively, the authors compared success rates between Fresh-ET after ovarian stimulation and Freeze/All-ET after artificial endometrial preparation - to compare endometrial receptivity. A two-stage, two-sided group sequential procedure with an overall type I error of.05 was used, to test the primary hypothesis of a difference in the probabilities of clinical pregnancy for the two arms in this study, with a maximum sample size of 411 patients needed to achieve 80% power for detecting a difference of 15% in the clinical pregnancy rate (sample size not reached). Patients were randomized by drawing randomly among identical, opaque, unmarked sealed envelopes (there was no blind description). The two groups were similar in age, diagnosis, baseline serum FSH level, antral follicle count, days of stimulation (10.5 versus 10.4), mean number of oocytes retrieved (12.9±4.7 for Freeze/All-ET group and 14.1±6.4 for Fresh-ET group), etc. Both groups did not differ significantly in number of transferred blastocyst or endometrial thickness on the trigger day. There were no significantly greater rates of clinical pregnancy per randomized patient (60.0% versus 43.3%), ongoing pregnancy per randomized patient (55.7% versus 40.3%), and no significant lower miscarriage rate from clinical pregnancy (14.3% versus 24.1%) in the Freeze/All-ET group. Patients with extreme high responses were taken off the study.
Chen et al., 2016: assessed 1,508 infertile women with PCOS, who were randomized during their first IVF cycle to undergo either Fresh-ET (n=762) or Freeze/All-ET (n=746). The patients were randomly assigned to one of the two study groups in a 1:1 ratio, using an online central randomization system, which was unknown to the clinical investigators. Both groups had similar IVF cycle characteristics, including age, endometrial thickness, days of stimulation (10.3 versus 10.3) and number of oocytes retrieved (14.4±6.0 for the Freeze/All-ET group and 14.2±5.8 for the Fresh-ET group). They found that the Freeze/All-ET group achieved significantly higher live births rate (49.3% versus 42.0%); higher, but not significant, clinical pregnancy rates (58.7% versus 56.2%) and ongoing pregnancy rates (52.7% versus 48.8%). On the other hand, miscarriage rates (from clinical pregnancies) were significantly lower in the Freeze/All-ET group (14.6% versus 25.0%). The authors also compared perinatal outcomes.
Vuong et al., 2016: In this randomized study, the aim was to compare the effectiveness of the Freeze/All-ET to conventional Fresh-ET in non-PCOS women. The inclusion criteria were: had ≤1 previous IVF cycle, could have embryo transfer on day 3, had at least 1 top-quality embryo. On the other hand, the exclusion criteria were: PCOS and oocyte donation. The days of stimulation were similar between the Freeze/All-ET and the Fresh-ET groups (9.16 versus 9.14). Randomization (1:1) was made by a computer-generated list. The sample size of 780 patients needed to achieve an 80% power for detecting a difference of 10% in ongoing pregnancy rates (there was no blind description). A total of 782 patients were included (391 in the Freeze/All-ET group and 391 in the Fresh-ET group). The primary outcome was ongoing pregnancy rates after the first embryo transfer. The baseline characteristics were similar between the groups, including age, stimulation duration and number of oocytes retrieved (12.6±5.6 for the cryopreservation group and 12.9±5.16 for the fresh group). They found no difference between the Freeze/All-ET and the Fresh-ET groups regarding ongoing pregnancy rates (36.3% versus 34.5%, respectively).
Coates et al., 2017: In this clinical trial, the aim was to identify which embryo transfer strategy, after preimplantation genetic screening (PGS) by next generation sequencing (NGS), freeze-all or Fresh-ET, would improve clinical outcomes or whether the strategies were equally successful. Women between the ages of 18 and 42 years, while undergoing IVF and PGS using their own eggs, were eligible to participate in the trial. The exclusion criteria included a need to use surgically retrieved sperm, patients using preimplantation genetic diagnosis for a single-gene or chromosomal disorder, egg donor cycles, gender selection cycles, decreased ovarian reserve (early follicular phase serum FSH level >10IU/L or random serum anti-Mullerian hormone level <1ng/ml), and any medical conditions occurring before recruitment. A total of 179 patients were randomized to either a Freeze/All-ET cycle (91) or a Fresh-ET (88) on day 6 during the stimulated cycle. A professional third party prepared the stratified block randomization sequence. The allocation sequence was stratified for female age (<35, 35-37, 38-40, and 41-42 years) and number of prior ART cycles (≤2 or ≥3). The women were randomized in a 1:1 ratio. The two groups were similar in age, anti-Mullerian hormone levels, FSH levels, mean number of oocytes retrieved (17.0 for Freeze/All-ET group and 14.0 for Fresh-ET group), etc. Frozen ETs were performed in an artificial cycle, and Fresh-ET were carried out during original egg retrieval cycle. The outcome of patients in the intention-to-treat analysis were: ongoing pregnancy rates (40.9% vs. 62.2%; p<0.1) and live birth rates (39.8 vs. 61.5%; p<0.1) per intended treatment was significantly higher for the freeze-all group compared with the fresh group.
Quality assessment
The methodological quality systems differ among the 5 RCTs. One trial did not have its complete data evaluated on methods ("non-randomized", "not-blind", "no-power calculation" descriptions) (Shapiro et al., 2011a). Randomization was done by drawing randomly among identical, opaque, unmarked sealed envelopes in one study (Shapiro et al., 2011b). In one study, the patients were randomly assigned to one of the two study groups in a 1:1 ratio, by an online central randomization system, which was unknown to the clinical investigators (Chen et al., 2016). Drawing randomly (1:1) was made by a computer-generated list in one study (Vuong et al., 2016). In one trial, a professional third party prepared the stratified block randomization sequence, and the allocation sequence was stratified for female age (Coates et al., 2017). Two studies described the method of blinding (Chen et al., 2016; Coates et al., 2017).
Main outcomes
The results of this meta-analysis were broken down into two parts, in accordance with the mean number of oocytes retrieved:
I- Outcomes when >12 to <21 oocytes retrieved
Clinical pregnancy rates (Figure 2)
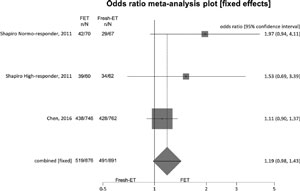
Figure 2. Clinical pregnancy rates when >12 and <21 oocytes were retrieved.
To analyze clinical pregnancy rates (per randomized patient), 3 studies were included, and there were no significant differences between the Fresh-ET group: 55.1%, (491/891) and the Freeze/All-ET group: 59.2% (519/876) (OR=1.19; 95%CI=0.98-1.43; p=0.09). There was no significant heterogeneity in this comparison: I2=33.2%; Cochran Q=2.99, p=0.22.
Ongoing pregnancy rates (Figure 3)
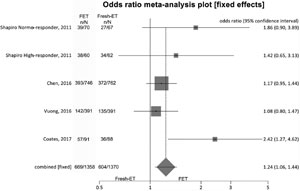
Figure 3. Ongoing pregnancy rates when >12 and <21 oocytes were retrieved.
To analyze ongoing pregnancy rates (per randomized patient), we included 5 studies and achieved significant differences between the groups: Fresh-ET: 44.1% (604/1370) versus Freeze/All-ET: 49.3% (669/1358) (OR=1.24; 95%CI=1.06-1.44; p=0.006). There was no significant heterogeneity in this comparison: I2=46.5%; Cochran Q=7.4, p=0.11.
Live birth rates (Figure 4)
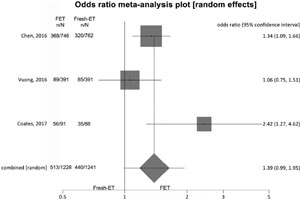
Figure 4. Live birth rates when >12 and <21 oocytes were retrieved.
To analyze live birth rates (per randomized woman) we included 3 trials and no significant difference was found between the groups: Fresh-ET: 35.5% (440/1241) versus Freeze/All-ET: 41.8% (513/1228) (OR=1.39; 95%CI=0.99-1.95; p=0.06). There was an important heterogeneity in this comparison: I2 =64.1%; Cochran Q=5.6; p=0.06.
Miscarriage rates
To analyze the rate of miscarriage (from clinical pregnancy), we considered 3 trials, and no significant difference was found between the groups: Fresh-ET: 13.8% (68/491) versus Freeze/All-ET: 10.0% (52/519) (OR=0.68; 95%CI=0.46-1.00; p=0.06). There was no heterogeneity in this comparison: I2=0%; Cochran Q=0.21, p=0.90.
II- Outcomes when >12 to <15 oocytes retrieved
Clinical pregnancy rates (Figure 5)
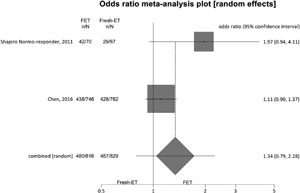
Figure 5. Clinical pregnancy rates when > 12 and < 15 oocytes were retrieved.
For clinical pregnancy rates (per randomized patient) we included 2 studies, and no significant difference was found between the fresh and the cryopreservation groups: Fresh-ET group: 55.1% (457/829) versus Freeze/All-ET group: 58.8% (480/816) (OR=1.34; 95%CI=0.79-2.28; p=0.27). The heterogeneity was measured: I2=NA; Cochran Q=2.5, p=0.11.
Ongoing pregnancy rates (Figure 6)
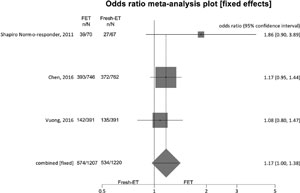
Figure 6. Ongoing pregnancy rates when >12 and <15 oocytes were retrieved.
For ongoing pregnancy rates (per randomized patient), we included 3 studies, and no significant difference was found between the groups: Fresh-ET group: 43.7% (534/1220) versus Freeze/All-ET group: 47.5% (574/1207) (OR=1.17; 95%CI=1.00-1.38; p=0.06). There was no significant heterogeneity in this comparison: I2=4.1%; Cochran Q=2.1, p=0.4.
Live birth rates (Figure 7)
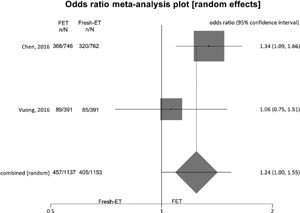
Figure 7. Live birth rates when >12 and <15 oocytes were retrieved.
To analyze live birth rates (per randomized woman) we included 2 studies, and no significant difference was found between the groups: Fresh-ET: 35.5% (405/1153) versus Freeze/All-ET: 41.8% (457/1137) (OR=1.24; 95%CI=1.00-1.55; p=0.05). The heterogeneity was measured: I2= NA; Cochran Q=1.4; p=0.2.
Miscarriage rates
For miscarriage rates (from clinical pregnancy) we included 3 studies, and no significant difference was found between the groups: Fresh-ET group: 13.8% (63/457) versus Freeze/All-ET group: 10.0% (48/480) (RR=0.68; 95%CI=0.46-1.02; p=0.06). The heterogeneity was measured: Cochran Q=0.2, p=0.65. A summary of the results of the present meta-analysis comparing Freeze/All-ET and Fresh-ET strategies is depicted on Table 2, including all trials (when the mean number of oocytes collected was >12 and <21), and on Table 3, including trials with the mean number of oocytes retrieved between >12 and <15.
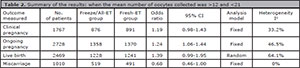
Table 2. Summary of the results: when the mean number of oocytes collected was >12 and <21
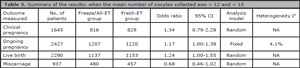
Table 3. Summary of the results: when the mean number of oocytes collected was > 12 and < 15
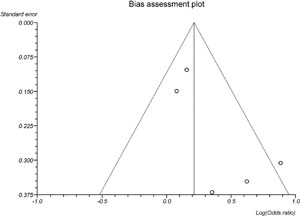
Publication bias
In the present meta-analysis concerning the freeze-all versus the fresh embryo transfers strategies, the publication biases were evaluated by Begg-Mazumdar (p=0.82), and Egger's tests (p=0.12). Visual inspection of Begg's funnel plots is available in the S1 Figure.
DISCUSSION
When there are uncertainties about a given medical question, a meta-analysis is an important tool, able to dissolve such problem. This analytical method consists of an approach in which different and independent studies are joined and the results are combined into a single common outcome. Compared with narrative reviews, meta-analyses have the great advantage of being less influenced by a reviewer's opinion, thus providing unbiassed conclusions. In addition, all the results can easily be recalculated and compared with the conclusions stated by the authors. Regarding endometrial receptivity, several procedures are being proposed to improve clinical outcomes in patients undergoing ART cycles, and the freeze-all strategy seems to be an important step in this direction (Shapiro et al., 2008; Shapiro et al., 2011a, 2011b; Chen et al., 2016; Vuong et al., 2016; Coates et al., 2017). This systematic review demonstrated that compared with Fresh-ET, the Freeze/All-ET brought about significant improvements to the ongoing pregnancy rates of patients submitted to ART procedures, when the mean number of oocytes collected was not limited to 15, regardless of having patients with PCOS. However, the Freeze/All-ET does not bear advantages when compared with Fresh-ET, when the mean number of oocytes retrieved is less than 15. These findings may be associated with the deleterious effects of COS on endometrial receptivity during ART cycles (Shapiro et al., 2011a; Chen et al., 2016).
There are several reasons that justify the employment of the freeze-all strategy, such as risk of ovarian hyperstimulation syndrome (OHSS), inadequate endometrial thickness, previous assisted reproduction procedure failures, infertility related to endometriosis, and high risk of venous thrombosis during ART procedures. However, the main pathophysiologic mechanism involved in the selection of the freeze-all strategy seems to be a premature progesterone elevation during COS, resulting in an impaired-reception uterine environment (Shapiro et al., 2011b; Mohamed et al., 2011; Nelson, 2013).
There is evidence in the literature to support this negative relationship between COS and pregnancy rates, probably due to the presence of elevated serum P and E levels during the follicular phase, promoting premature luteinization (PL), which occurrence is seen in up to 30% of IVF/ICSI cycles (Schoolcraft et al., 1991; Fanchin et al., 1993; Givens et al., 1994; Venetis et al., 2013). Possible explanations for PL occurrence, could be associated with the rising levels of E, that may induce increased LH secretion, able to stimulate granulosa cells to produce progesterone but unable to promote trigger ovulation (Ubaldi et al., 1995; Melo et al., 2006), and increases in the number of mature follicles with 17mm or more (Peluso, 1990; Bosch et al., 2003; Glamočlija et al., 2005). In addition, increased concentration of estrogen during the follicular phase in COS, upregulates endometrial progesterone receptor expression in comparison with what happens in natural cycles, promoting advanced endometrial maturation (Koo et al., 2015). The success of ART cycles is dependent on the number and quality of oocytes and embryos, and endometrial receptivity (Schoolcraft et al., 1991; Kagawa et al., 1992; Silverberg et al., 1994; Sims et al., 1994; Bosch et al., 2003; Lai et al., 2009; Milachich & Shterev, 2016). The main negative effect of P elevation during ART procedures seems to be on endometrial receptivity (endometrial asynchrony), rather than on oocyte or embryo quality (Lu et al., 2016). This harmful effect of P elevation on endometrial receptivity in patients undergoing fresh autologous IVF/ICSI cycles becomes more evident knowing that the highest pregnancy rates occur in fresh oocyte donation cycles, wherein the endometrium is artificially prepared, without deleterious COS effects (Legro et al., 1993; Silverberg et al., 1994; Shapiro et al., 2009).
In view of the plausible negative effects of COS, mainly in high-responders, it has been demonstrated that the Freeze/All-ET could be the better choice to improve clinical outcomes in patients with higher P levels (Shapiro et al., 2011a; Lu et al., 2016). However, Levi and collaborators (in a non-randomized study) suggested that in patients submitted to ART procedures with a mean number of oocytes collected greater than 15, COS did not result in damage on endometrial receptivity, and the relative brief COS with reduced number of days of ovarian stimulation (8.4 days) could explain the reduced negative endometrial effect (Levi et al., 2001).
On the other hand, the RCTs on freeze-all strategy available in the literature do not differ vis-à-vis outcomes involving ongoing pregnancy rates per randomized patient in the group of women with a mean number of retrieved oocytes below 15. Shapiro and collaborators demonstrated, in a prior RCT involving normal-responders, that ongoing pregnancy rates (per patient) was not higher in the group submitted to Freeze/All-ET, when compared with the group of patients in whom Fresh-ET was performed (Shapiro et al., 2011b). Also, agreeing with the outcomes of this systematic review, Vuong and collaborators showed that patients with a mean number of collected oocytes of approximately 13, did not benefit from the Freeze/All-ET (Vuong et al., 2016). Similarly, Chen and collaborators reported that the Freeze/All-ET group achieved higher, but not significant ongoing pregnancy rates (per patient) (Chen et al., 2016).
A meta-analysis is a powerful tool, considered the highest in the evidence-based pyramid, but its strength depends on the quality of the randomized trials analyzed (Franco & Oliveira, 2015). Recently, an RCT including high-responders, favoring the Freeze/All-ET strategy was retracted of the literature access (Aflatoonian et al., 2010). This retracted RCT is part of a relatively recent meta-analysis (Roque et al., 2013) concerning the beneficial effects of cryopreservation and subsequent FET. However, removing the aforementioned study, the prior meta-analysis (Roque et al., 2013) loses its power to assist in medical decision-making whether the Freeze/All-ET should be used or not in clinical practice.
The freeze-all strategy is a topic that has recently gained attention from clinicians and embryologists. However, although it has great relevance for advances in ART, more prospective and randomized trials, involving large populations are necessary to define whether delayed frozen-thawed embryo transfer is beneficial, and for which groups of patients it could provide improvements in the clinical outcomes of IVF/ICSI cycles.
In conclusion, the findings of this meta-analysis suggest that the freeze-all strategy could be favorable when high numbers of oocytes are collected, signaling an association between higher COS and consequent impairment in endometrial receptivity. However, when the mean number of oocytes collected is less than 15, the freeze-all strategy does not appear to be advantageous. More RCTs are required to evaluate whether the freeze-all strategy could influence clinical outcomes.
REFERENCES
Aflatoonian A, Oskouian H, Ahmadi S, Oskouian L. Can fresh embryo transfers be replaced by cryopreserved-thawed embryo transfers in assisted reproductive cycles? A randomized controlled trial. J Assist Reprod Genet. 2010;27:357-63. Retraction in: J Assist Reprod Genet. 2013;30:1245. PMID: 23975193 DOI: 10.1007/s10815-013-0084-0
Medline Crossref
Bosch E, Valencia I, Escudero E, Crespo J, Simón C, Remohí J, Pellicer A. Premature luteinization during gonadotropin-releasing hormone antagonist cycles and its relationship with in vitro fertilization outcome. Fertil Steril. 2003;80:1444-9.
Medline Crossref
Chen ZJ, Shi Y, Sun Y, Zhang B, Liang X, Cao Y, Yang J, Liu J, Wei D, Weng N, Tian L, Hao C, Yang D, Zhou F, Shi J, Xu Y, Li J, Yan J, Qin Y, Zhao H, Zhang H, Legro RS. Fresh versus Frozen Embryos for Infertility in the Polycystic Ovary Syndrome. N Engl J Med. 2016;375:523-33.
Medline Crossref
Coates A, Kung A, Mounts E, Hesla J, Bankowski B, Barbieri E, Ata B, Cohen J, Munné S. Optimal euploid embryo transfer strategy, fresh versus frozen, after preimplantation genetic screening with next generation sequencing: a randomized controlled trial. Fertil Steril. 2017;107:723-30.e3.
Medline Crossref
Fanchin R, de Ziegler D, Taieb J, Hazout A, Frydman R. Premature elevation of plasma progesterone alters pregnancy rates of in vitro fertilization and embryo transfer. Fertil Steril. 1993;59:1090-4.
Medline Crossref
Franco JG Jr, Oliveira JB. The Impact of Meta-analyses on Medical Decisions. JBRA Assist Reprod. 2015;19:109-10.
Medline Crossref
Givens CR, Schriock ED, Dandekar PV, Martin MC. Elevated serum progesterone levels on the day of human chorionic gonadotrophin administration do not predict outcome in assisted reproduction cycles. Fertil Steril. 1994;62:1011-7.
Medline Crossref
Glamočlija V, Vilović K, Saraga-Babić M, Baranović A, Sapunar D. Apoptosis and active caspase-3 expression in human granulosa cells. Fertil Steril. 2005;83:426-31.
Medline Crossref
Griesinger G, von Otte S, Schroer A, Ludwig AK, Diedrich K, Al-Hasani S, Schultze-Mosgau A. Elective cryopreservation of all pronuclear oocytes after GnRH agonist triggering of final oocyte maturation in patients at risk of developing OHSS: A prospective, observational proof-of-concept study. Hum Reprod. 2007;22:1348-52.
Medline Crossref
Griesinger G, Schultz L, Bauer T, Broessner A, Frambach T, Kissler S. Ovarian hyperstimulation syndrome prevention by gonadotropin-releasing hormone agonist triggering of final oocyte maturation in a gonadotropin-releasing hormone antagonist protocol in combination with a "freeze-all" strategy: a prospective multicentric study. Fertil Steril. 2011;95:2029-33, 2033.e1.
Medline Crossref
Huang Y, Wang EY, Du QY, Xiong YJ, Guo XY, Yu YP, Sun YP. Progesterone elevation on the day of human chorionic gonadotropin administration adversely affects the outcome of IVF with transferred embryos at different developmental stages. Reprod Biol Endocrinol. 2015;13:82.
Medline Crossref
Kagawa T, Yamano S, Nishida S, Murayama S, Aono T. Relationship among serum levels of luteinizing hormone, estradiol, and progesterone during follicle stimulation and results of in vitro fertilization and embryo transfer (IVF-ET). J Assist Reprod Genet. 1992;9:106-12.
Medline Crossref
Kansal Kalra S, Ratcliffe SJ, Milman L, Gracia CR, Coutifaris C, Barnhart KT. Perinatal morbidity after in vitro fertilization is lower with frozen embryo transfer. Fertil Steril. 2011;95:548-53.
Medline Crossref
Kolibianakis E, Bourgain C, Albano C, Osmanagaoglu K, Smitz J, Van Steirteghem A, Devroey P. Effect of ovarian stimulation with recombinant follicle-stimulating hormone, gonadotropin releasing hormone antagonists, and human chorionic gonadotropin on endometrial maturation on the day of oocyte pick-up. Fertil Steril. 2002;78:1025-9.
Medline Crossref
Koo HS, Cha SH, Kim HO, Song IO, Min EG, Yang KM, Park CW. A high response to controlled ovarian stimulation induces premature luteinization with a negative impact on pregnancy outcomes in a gonadotropin-releasing hormone antagonist cycle. Clin Exp Reprod Med. 2015;42:149-55.
Medline Crossref
Lai TH, Lee FK, Lin TK, Horng SG, Chen SC, Chen YH, Wang PC. An increased serum progesterone-to-estradiol ratio on the day of human chorionic gonadotropin administration does not have a negative impact on clinical pregnancy rate in women with normal ovarian reserve treated with a long gonadotropin releasing hormone. Fertil Steril. 2009;92:508-14.
Medline Crossref
Legro RS, Ary BA, Paulson RJ, Stanczyk FZ, Sauer MV. Premature luteinization as detected by elevated serum progesterone is associated with a higher pregnancy rate in donor oocyte in-vitro fertilization. Hum Reprod. 1993;8:1506-11.
Medline Crossref
Levi AJ, Drews MR, Bergh PA, Miller BT, Scott RT Jr. Controlled ovarian hyperstimulation does not adversely affect endometrial receptivity in in vitro fertilization cycles. Fertil Steril. 2001;76:670-4.
Medline Crossref
Lu X, Chen Q, Fu Y, Ai A, Lyu Q, YP K. Elevated progesterone on the trigger day does not impair the outcome of Human Menotrophins Gonadotrophin and Medroxyprogesterone acetate treatment cycles. Sci Rep. 2016;6:31112.
Medline Crossref
Melo MA, Meseguer M, Garrido N, Bosch E, Pellicer A, Remohí J. The significance of premature luteinization in an oocyte-donation programme. Hum Reprod. 2006;21:1503-7.
Medline Crossref
Milachich T, Shterev A. Are there optimal numbers of oocytes, spermatozoa and embryos in assisted reproduction? JBRA Assist Reprod. 2016;20:142-9.
Medline Crossref
Mohamed AM, Chouliaras S, Jones CJ, Nardo LG. Live birth rate in fresh and frozen embryo transfer cycles in women with endometriosis. Eur J Obstet Gynecol Reprod Biol. 2011;156:177-80.
Medline Crossref
Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(7): e1000097 For more information, visit: www.prisma-statement.org
Medline Crossref
Murata Y, Oku H, Morimoto Y, Tokuda M, Murata T, Sugihara K, Nagata F, Nakaoka Y, Fukuda A. Freeze-thaw programmes rescue the implantation of day 6 blastocysts. Reprod Biomed Online. 2005;11:428-33.
Medline Crossref
Nelson SM. Venous thrombosis during assisted reproduction: Novel risk reduction strategies. Thromb Res. 2013;131:S1-3.
Medline Crossref
Nikas G, Develioglu OH, Toner JP, Jones HW Jr. Endometrial pinopodes indicate a shift in the window of receptivity in IVF cycles. Hum Reprod. 1999;14:787-92.
Medline Crossref
Peluso JJ. Role of the amplitude of the gonadotropin surge in the rat. Fertil Steril. 1990;53:150-4.
Medline Crossref
Richter KS, Shipley SK, McVearry I, Tucker MJ, Widra EA. Cryopreserved embryo transfers suggest that endometrial receptivity may contribute to reduced success rates of later developing embryos. Fertil Steril. 2006;86:862-6.
Medline Crossref
Roque M, Lattes K, Serra S, Solà I, Geber S, Carreras R, Checa MA. Fresh embryo transfer versus frozen embryo transfer in in vitro fertilization cycles: A systematic review and meta-analysis. Fertil Steril. 2013;99:156-62.
Medline Crossref
Schoolcraft W, Sinton E, Schlenker T, Huynh D, Hamilton F, Meldrum DR. Lower pregnancy rate with premature luteinization during pituitary suppression with leuprolide acetate. Fertil Steril. 1991;55:563-6.
Medline Crossref
Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Thomas S. Large blastocyst diameter, early blastulation, and low preovulatory serum progesterone are dominant predictors of clinical pregnancy in fresh autologous cycles. Fertil Steril. 2008;90:302-9.
Medline Crossref
Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C, Thomas S. High ongoing pregnancy rates after deferred transfer through bipronuclear oocyte cryopreservation and post-thaw extended culture. Fertil Steril. 2009;92:1594-9.
Medline Crossref
Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C, Thomas S. Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: a prospective randomized trial comparing fresh and frozen-thawed embryo transfers in high responders. Fertil Steril. 2011a;96:516-8.
Medline Crossref
Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C, Thomas S. Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: a prospective randomized trial comparing fresh and frozen-thawed embryo transfer in normal responders. Fertil Steril. 2011b;96:344-8.
Medline Crossref
Silverberg KM, Martin M, Olive DL, Burns WN, Schenken RS. Elevated serum progesterone levels on the day of human chorionic gonadotropin administration in in vitro fertilization cycles do not adversely affect embryo quality. Fertil Steril. 1994;61:508-13.
Medline Crossref
Sims JA, Seltman HJ, Muasher SJ. Early follicular rise of serum progesterone concentration in response to a flare-up effect of gonadotrophin-releasing hormone agonist impairs follicular recruitment for in-vitro fertilization. Hum Reprod. 1994;9:235-40.
Medline Crossref
Ubaldi F, Smitz J, Wisanto A, Joris H, Schiettecatte J, Derde MP, Borkham E, Van Steirteghem A, Devroey P. Oocyte and embryo quality as well as pregnancy rate in intracytoplasmic sperm injection are not affected by high follicular phase serum progesterone. Hum Reprod. 1995;10:3091-6.
Medline Crossref
Venetis CA, Kolibianakis EM, Bosdou JK, Tarlatzis BC. Progesterone elevation and probability of pregnancy after IVF: a systematic review and meta-analysis of over 60 000 cycles. Hum Reprod Update. 2013;19:433-57.
Medline Crossref
Vuong LT, Dang VQ, Ho TM, Huynh BG, Ha DT, Pham TD, Nguyen LK, Norman RJ, Mol BW. Freeze-all versus fresh embryo transfer in IVF/ICSI, a randomised controlled trial. Fertil Steril. 2016;106:e376.
Crossref
Wong KM, Mastenbroek S, Repping S. Cryopreservation of human embryos and its contribution to in vitro fertilization success rates. Fertil Steril. 2014;102:19-26.
Medline Crossref