JBRA Assist. Reprod. 2014; 18 (3):80-84
ORIGINAL ARTICLE
doi: 10.5935/1518-0557.20140012
Anti-Mullerian as predictor of reproductive outcome in infertile women: follow up
1IBRRA - Instituto Brasileiro de Reprodução Assistida, Belo Horizonte, MG, Brazil
2Department of Reproductive Medicine, Hôpital Jean Verdier (AP-HP), University Paris XIII, and INSERM, U782, Clamart
CONFLICT OF INTERESTS
The authors declare that they have no conflict of interest.
ABSTRACT
Objective: The aim of the present study was to investigate and to compare the relations of anti-Mullerian with the prognostic parameters and the outcome of assisted reproductive treatment.
Methods: Prospective longitudinal study. A total of one hundred and twelve infertile women. Inclusion criteria: i) both ovaries present, ii) no current or past diseases affecting ovaries or gonadotropin or sex steroid secretion, clearance, or excretion, iii) no current hormone therapy, iv) adequate visualization of ovaries at transvaginal ultrasound scans, and v) total number of small antral follicles (3–12 mm in diameter) between 1 and 32 follicles. On cycle day 3, woman underwent blood sampling for serum FSH and AMH measurement and a transvaginal ovarian ultrasound scan for follicle measurement. Ongoing pregnancy was evaluated as biochemical pregnancy and observation of gestational sac(s).
Results: Mean age of 36.13 ± 4.65 years old, BMI 21.59 ± 2.78 kg/m2, and length of infertility of 2.88 ± 2.36 years. Their ovaries had an average of 13.74 ± 6.0 antral follicles and AMH was 2.49 ± 1.98 ng / mL. A significant relationship of AMH with age (r = -0.37 P <.01) , with FSH (r = -0.22, P <.01) , with AFC (r = 0.74, P <.00001), with smoking (P <.009), with SOP (P <.00001), with the total dose of the drug during stimulation ovarian (r = -0.36, P <.0004), with abortion (P <.05) and with the ongoing pregnancy (P <.05).
Conclusion: AMH is a marker of quantitative and qualitative aspects of the ovarian reserve.
Keywords: AMH, FSH, antral follicles, ovarian reserve, ongoing pregnancy
INTRODUCTION
Anti-Müllerian hormone (AMH), also called Müllerian Inhibiting substance, is a dimeric glycoprotein, belonging to the transforming growth factor-b (TGF-b) superfamily, such as activins and inhibins, and is produced exclusively in the gonads, as shown more than two decades ago in animals (Vigier et al., 1984) and later in humans (Rey et al., 2003; Di Clemente et al., 1992). In women, AMH is synthetized by the granulosa cells (GC) surrounding preantral and small antral follicles (Weenen et al., 2004; Durlinger et al., 2002). Despite the use of ultrasensitive assays, AMH is barely detectable in the serum at birth. It reaches higher levels after puberty (Guibourdenche et al., 2003; Rajpert-De Meyts et al., 1999) and then declines with advancing female age, until becoming undetectable again at the time of the menopause (La Marca et al., 2005). Although its physiological roles and the mechanisms involved in the regulation of AMH still remain poorly established, recent studies have pointed out this hormone as an attractive marker for assessing of ovarian activity.
Basal AMH, determined before stimulation (usually cycle day 3), was found to be a better measure for the assessment of a decreased ovarian reserve and the ovarian response to controlled ovarian hyperstimulation (COH) with gonadotropins (Anckaert et al., 2012; Nardo et al., 2009) when compared to the classic parameters such as an increase in follicle stimulating hormone (FSH), the decrease of inhibin B, or the antral follicle count (Fanchin et al., 2005; Tremellen et al., 2005; Hazout, 2006). It has also been shown that AMH was inversely correlated, in addition to age, to basal FSH values (Piltonen et al., 2005). AMH has been claimed to possess at least the same level of accuracy as the antral follicle count (AFC) for the prediction of poor and excessive (Broer et al., 2011) response. In addition, a high serum concentration of AMH before the start of COH has been shown to be associated with increased risk of developing ovarian hyperstimulation syndrome (OHSS) (Nardo et al., 2009). As with other ovarian reserve tests, AMH has not proven to be a good predictor of embryo quality or pregnancy in COS cycles, suggesting that AMH is a marker of quantitative rather than qualitative aspects of the ovarian reserve (Anckaert et al., 2012; Broer et al., 2011).
The association between the ovarian response and the basal AMH production was confirmed by other studies, showing an increased yield of mature oocytes after controlled stimulation in women presenting high serum AMH levels (Fleming et al., 2006; Smeenk et al., 2007; Ebner et al., 2006; Ficicioglu et al., 2006).
The aim of the present study was to investigate and to compare the relations of AMH with the prognostic parameters and the outcome of assisted reproductive technology (ART).
MATERIAL AND METHODS
Subjects
One hundred and twelve infertile women, aged 22-50 years, undergoing routine exploration during an unstimulated cycle that preceded ART at our center were studied prospectively, from February 2009 to December 2012. All patients met the following inclusion criteria: i) both ovaries present, ii) no current or past diseases affecting ovaries or gonadotropin or sex steroid secretion, clearance, or excretion, iii) no current hormone therapy, iv) adequate visualization of ovaries at transvaginal ultrasound scans, and v) total number of small antral follicles (3–12 mm in diameter) between 1 and 32 follicles, including both ovaries. All patients signed an informed consent form for this analysis.
Protocol
The patients received leuprolide acetate (Lupron, Abbott, France), the GnRH-agonist was initiated at a dose of 2,0 mg per day during the midluteal phase with approximately a 5-day overlap with the OCP (Diane 35, Schering, Brasil). Pituitary down-regulation was monitored and patients with adequate pituitary desensitization started their recombinant FSH regime (Gonal-F; Merck-Serono Pharmaceuticals, Italy) and the dose of GnRH-agonist was reduced to 1.0 mg per day. FSH was started with dosages between 150 and 300 IU daily for 4 days with or without human menopausal gonadotropin (hMG) (Menopur; Ferring Pharmaceuticals, Germany). There after, the dose of FSH was individually adjusted according to the estradiol (E2) response and vaginal ultrasound findings.
When two follicles reached ≥16 to 18 mm, 250 mg, recombinant human Chorionic Gonadotropin (Ovidrel, Merck-Serono Pharmaceuticals, Italy) was administered and oocyte retrieval occurred 35 to 36 hours later.
Intracytoplasmatic sperm Injection (ICSI) was routinely performed in all the fertilization procedures. Fertilization was evident when two pronuclei were observed. Embryos were cultured until the day of transfer (day 3) in IVF Global® media (Life Global, Canada) supplemented with 10 % synthetic serum substitute (SSS) and graded by Veeck’s (Veeck, 1999) and Hsu (Hsu et al., 1999) criteria before transfer.
The ET number was determined by following the Federal Council of Medicine – Brazil (FCM) guidelines.
Luteal phase was supported with micronized P4, 600 mg/day, administered continuously by vaginal route, starting on the evening of ET.
Ongoing pregnancy (OP) was evaluated as biochemical pregnancy (BQ) and subsequent observation of gestational sac(s). Abortion defined as a clinically recognized pregnancy loss before 20 weeks’ gestation.

Figure 1. (A) Correlation between Anti-Mullerian Hormone (AMH) and age (B) Correlation between Anti-Mullerian (AMH) and day-3 Follicle Stimulating Hormone level (FSH).
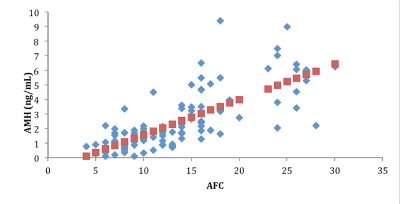
Figure 2. Correlation between Anti-Mullerian (AMH) and Antral Follicle Count (AFC).
Hormonal Measurements and Ultrasound Scans
On cycle day 3 of the cycle preceding COH, each woman underwent blood sampling by venipuncture for serum AMH, and FSH measurement and a transvaginal ovarian ultrasound scan for follicle measurement.
Serum levels of AMH and FSH were determined using an automated multianalysis system with chemiluminescence detection (ACS-180; Bayer Diagnostics, Puteaux, France). Serum AMH levels were determined using a second generation enzyme-linked immunosorbent assay. Intra- and inter- assay coefficients of variation (CV) were < 6 and <10% respectively, lower detection limit at 0,13 ng/mL and linearity up to 21 ng/mL for AMH.
For FSH, functional sensitivity was 0.1 mIU/mL, and intra-assay and interassay CV were 3% and 5%, respectively.
Ultrasound scans were performed using a 3.7–9.3MHz multifrequency transvaginal probe (RIC5-9H; General Electric Medical Systems, Paris, France) by a single operator who was blinded to the results of hormone assays.
The objective of ultrasound examination was to evaluate the number and size of small antral follicles. Follicles measuring 3–12 mm in mean diameter (mean of two orthogonal diameters) in both ovaries were considered.
To optimize the reliability of ovarian follicular assessment, the ultrasound scanner was equipped with a tissue harmonic imaging system (Thomas & Rubin, 1998), which allowed improved image resolution and adequate recognition of follicular borders.
Intra-analysis CV for follicular and ovarian measurements were <5%, and their lower limit of detection was 0.1 mm. In an effort to evaluate the bulk of granulosa cells in both ovaries, we calculated the mean follicle diameter (cumulative follicle diameter divided by the number of follicles measuring 3–12 mm in diameter in both ovaries) and the largest follicle diameter.
Ethical approval
Written informed consent was obtained from all participants before inclusion. The study was approved by IBRRA Ethical Committee.
Statistical Analysis
Descriptive parameters and patient characteristics were reported as mean SD or median (range) depending on the distribution.
The Student’s t-test was performed for continuous variables Wilcoxon and Pearson’s Test were used where appropriate for categorical variables. P <.05 was considered statistically significant.
RESULTS
Overall, at the time of the investigation, patients had a mean age of 36.13 ± 4.65 years old, BMI 21.59 ± 2.78 kg/m2, and length of infertility of 2.88 ± 2.36 years. 79% had regular cycles and 3.6% were smokers. On cycle day 3, serum AMH level was 2.49 ± 1.98 ng/mL. At baseline, women had 13.74 ± 6.0 antral follicles.
When the 112 patients evaluated was observed a significant relationship of AMH with age (r = -0.37 P <.01) (Fig. 1A), with FSH (r = -0.22, P <.01) (Fig. 1B), with AFC (r = 0.74, P <.00001) (Fig. 2), with smoking (P <.009), with SOP (P <.00001), with the total dose of the drug during stimulation ovarian (r = -0.36, P <.0004), with abortion (P <.05) (Fig. 3) and with the ongoing pregnancy (P <.05) (Fig. 4).
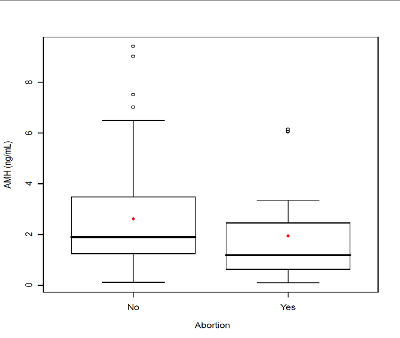
Figure 3. Serum anti-Mullerian hormone (AMH) comparison between infertile patients with abortion and infertile patients without abortion. The box represents the interquartile range that contains the 50% of values. The whiskers are line that extend from the box to highest and lowest values, exclude outliers. A line across the box indicates the median. P<.05, Student’s t-test.
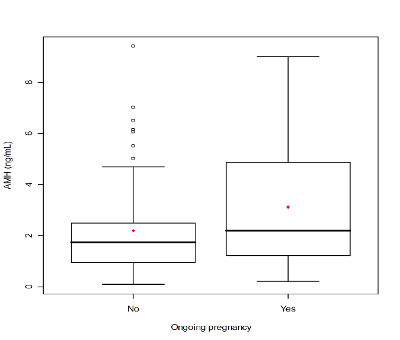
Figure 4. Serum anti-Mullerian hormone (AMH) comparison between infertile patients with ongoing pregnancy and infertile patients no ongoing pregnancy. The box represents the interquartile range that contains the 50% of values. The whiskers are line that extend from the box to highest and lowest values, exclude outliers. A line across the box indicates the median. P<.05, Student’s t-test.
DISCUSSION
This study demonstrates that serum AMH level is an independent predictor of ongoing pregnancy in infertile women.
Ovarian reserve is currently defined as an interplay between the quantity and quality of the follicles left in the ovary and several proxy variables for pool size are well described in the literature.
AMH can probably predict pregnancy, which is often used as a proxy for oocyte quality, is still a matter of debate (Ficicioglu et al., 2006; Lekamge et al., 2007; Broer et al., 2009).
An explanation for our finding that AMH level can predict ongoing pregnant in this cohort could be the fact that higher AMH values are associated with a higher oocyte yield in IVF treatment.
This higher oocyte yield consequently results in higher chances of pregnancy (Broer et al., 2011; Fleming et al., 2006).
Similarly, another possible explanation of our result is the positive correlation between the serum AMH level and embryo score quality (Silberstein et al., 2006) although another study (Lekamge et al., 2007) no correlation was found. It is also observed a correlation between miscarriages with serum levels of AMH (Elter et al., 2005) that can be explained by increased rate of fetal aneuploidy indirectly related to the embryo score.
This study does not itself explain the physiological basis of these findings. We postulate, however, that continued recruitment of additional antral follicles during the stimulatory phase of IVF results in higher AMH levels in individuals destined to produce better quality embryos and to have a better reproductive outcome.
Comparisons between the published data are therefore difficult to make due to their heterogeneity.
More research will be required to confirm this finding and to explore its etiology.
In our study, total consumption of the gonadotropin dose was statistically correlated with AMH.
The relationship between serum AMH levels and controlled ovarian stimulation outcome that we have observed is in agreement with previous studies on serum AMH levels.
Serum AMH seems to reflect the follicular pool, and its production is independent of the gonadotropin-dependent indicators of ovarian reserve.
Another correlation is demonstrated in this study of AMH with polycystic ovarian syndrome (PCOS).
The women with PCOS have elevated concentrations of AMH due probably an abnormal activity of the GCs, circulating androgen levels and the follicle excess seen on ultrasonographic examination (Laven et al., 2004; Pigny et al., 2006; Pellatt et al., 2007; Das et al., 2008; Li et al.,2011).
The negative impact of both male and female smoking on spontaneous and assisted conception rates has been largely demonstrated (Dechanet et al., 2011; Waylen et al., 2009). Tobacco consumption can result in an alteration of ovarian reserve, negative influence on the uterine receptiveness (Soares et al., 2007) and possibly on the fertilization and embryo development (Hassa et al., 2007; Jennings et al., 2011; Huang et al., 2009).
Concerning IVF cycles, at present, the majority of the studies available report that smokers experience lower ovarian response to COH (Neal et al., 2005; Weigert et al., 1999), and finally lower implantation rate (Dessolle et al., 2011).
CONCLUSION
AMH is a marker of quantitative and qualitative aspects of the ovarian reserve.
In summary, AMH is now our main method of determining ovarian reserve and selecting our pretreatment counseling and choice of infertility treatment.
We believe it to be the most informative serum marker available and that it should be considered an important part of any contemporary reproductive medicine practice.
Acknowledgements
We would like to thank Dr Michael Grynberg at Hôpital Antoine Béclère, France, for the critical reading of this manuscript.
REFERENCES
Anckaert E, Smitz J, Schiettecatte J, Klein B, Arce J-C. The value of anti-mullerian hormone measurement in the long GnRH agonist protocol: association with ovarian response, dose adjustments, embryo quality and pregnancy. Hum Reprod 2012;27:1829–39.
Medline Crossref
Broer SL, Mol BW, Hendriks D, Broekmans FJ. The role of antimullerian hormone in prediction of outcome after IVF: comparison with the antral follicle count. Fertil Steril 2009;91:705–14.
Medline Crossref
Broer SL, Dolleman M, Opmeer BC, Fauser BC, Mol BW, Broekmans FJ. AMH and AFC as predictors of excessive response in controlled ovarian hyperstimulation: a meta-analysis. Hum Reprod Update 2011;17:46–54.
Medline Crossref
Das M, Gillott DJ, Saridogan E, Djahanbakhch O. Anti-Mullerian hormone is increased in follicular fluid from unstimulated ovaries in women with polycystic ovary syndrome. Hum Reprod 2008;23:2122–6.
Medline Crossref
Dechanet C, Anahory T, Mathieu Daude JC, Quantin X, Reyftmann L, Hamamah S, Hedon B, Dechaud H. Effects of cigarette smoking on reproduction. Hum Reprod Update 2011;17:76-95.
Medline Crossref
Dessolle L, Freour T, Ravel C, Jean M, Colombel A, Darai E, Barriere P. Predictive factors of healthy term birth after single blastocyst transfer. Hum Reprod 2011;26:1220–6.
Medline Crossref
Di Clemente N, Ghaffari S, Pepinsky R, Pican C, Josso N, Cate RL, Vigier B. A quantitative and interspecific test for biological activity of anti-Mullerian hormone: the fetal ovary aromatase assay. Development 1992;114:721–7.
Medline
Durlinger AL, Gruijters MJ, Kramer P, Karels B, Ingraham HA, Nachtigal MW, Uilenbroek JT, Grootegoed JA, Themmen AP. Anti-Mullerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology 2002;143:1076–84.
Medline Crossref
Ebner T, Sommergruber M, Moser M, Shebl O, Schreier E, Tews G. Basal level of anti-Mullerian hormone is associated with oocyte quality in stimulated cycles. Hum Reprod 2006;21:2022–6.
Medline Crossref
Elter K, Kavak ZN, Gokaslan H, Pekin T. Antral follicle assessment after down-regulation may be a useful tool for predicting pregnancy loss in in vitro fertilization pregnancies. Gynecol Endocrinol 2005;21:33-7.
Medline Crossref
Fanchin R, Taieb J, Mendez DH, Ducot B, Frydman R, Bouyer J. High reproducibility of serum anti-Mullerian hormone measurements suggests a multi-staged follicular secretion and strengthens its role in the assessment of ovarian follicular status. Hum Reprod 2005;20:923–7.
Medline Crossref
Ficicioglu C, Kutlu T, Baglam E, Bakacak Z. Early follicular antimullerian hormone as an indicator of ovarian reserve. Fertil Steril 2006;85:592–6.
Medline Crossref
Fleming R, Deshpande N, Traynor I, Yates RW. Dynamics of FSH- induced follicular growth in subfertile women: relationship with age, insulin resistance, oocyte yield and anti-Mullerian hormone. Hum Reprod 2006;21:1436–41.
Medline Crossref
Guibourdenche J, Lucidarme N, Chevenne D, Rigal O, Nicolas M, Luton D, Léger J, Porquet D, Noel M. Anti-Mullerian hormone levels in serum from human foetuses and children: pattern and clinical interest. Mol Cell Endocrinol 2003;211:55–63.
Medline Crossref
Hassa H, Gurer F, Tanir HM, Kaya M, Gunduz NB, Sariboyaci AE, Bal C. Effect of cigarette smoke and alpha-tocopherol (vitamin E) on fertilization, cleavage, and embryo development rates in mice: an experimental in vitro fertilization mice model study. Eur J Obstet Gynecol Reprod Biol 2007; 135:177–82.
Medline Crossref
Hazout A. [Quality of ovarian reserve: inhibin B on day 3 of the cycle or antimüllerian hormone (AMH)?] Gynecol Obstet Fertil 2006;34:1001–2.
Medline Crossref
Huang J, Okuka M, McLean M, Keefe DL, Liu L. Effects of cigarette smoke on fertilization and embryo development in vivo. Fertil Steril 2009;92:1456–65.
Medline Crossref
Hsu MI, Mayer J, Aronshon M, Lanzendorf S, Muasher S, Kolm P, Oehninger S. Embryo implantation in in vitro fertilization and intracytoplasmic sperm injection: impact of cleavage status, morphology grade, and number of embryos transferred. Fertil Steril 1999;72:679 – 85.
Medline Crossref
Jennings PC, Merriman JA, Beckett EL, Hansbro PM, Jones KT. Increased zona pellucida thickness and meiotic spindle disruption in oocytes from cigarette smoking mice. Hum Reprod 2011;26:878–84.
Medline Crossref
La Marca A, De Leo V, Giulini S, Orvieto R, Malmusi S, Gianella L, Volpe A. Anti-Mullerian hormone in premenopausal women and after spontaneous or surgically induced menopause. J Soc Gynecol Invest 2005;12: 545–8.
Medline Crossref
Laven JS, Mulders AG, Visser JA, Themmen AP, De Jong FH, Fauser BC. Anti-Mullerian hormone serum concentrations in normoovulatory and anovulatory women of reproductive age. J Clin Endocrinol Metab 2004;89:318–23.
Medline Crossref
Lekamge DN, Barry M, Kolo M, Lane M, Gilchrist RB, Tremellen KP. Anti-Mullerian hormone as a predictor for IVF outcome. Reprod Biomed Online 2007;14:602–10.
Medline Crossref
Li HW, Anderson RA, Yeung WS, Ho PC, Ng EH. Evaluation of serum anti- mullerian hormone and inhibin B concentrations in the differential diagnosis of secondary oligoamenorrhea. Fertil Steril 2011;96:774–9.
Medline Crossref
Nardo LG, Gelbaya TA, Wilkinson H, Roberts SA, Yates A, Pemberton P, Laing I. Circulating basal anti-mullerian hormone levels as predictor of ovarian response in women undergoing ovarian stimulation for in vitro fertilization. Fertil Steril 2009;92:1586–93.
Medline Crossref
Neal MS, Hughes EG, Holloway AC, Foster WG. Sidestream smoking is equally as damaging as mainstream smoking on IVF outcomes. Hum Reprod 2005;20:2531–5.
Medline Crossref
Pellatt L, Hanna L, Brincat M, Galea R, Brain H, Whitehead S, Mason H. Granulosa cell production of anti-Mullerian hormone is increased in polycystic ovaries. J Clin Endocrinol Metab 2007;92:240–5.
Medline Crossref
Pigny P, Jonard S, Robert Y, Dewailly D. Serum anti-Mullerian hormone as a surrogate for antral follicle count for definition of the polycystic ovary syndrome. J Clin Endocrinol Metab 2006;91:941–5.
Medline Crossref
Piltonen T, Morin-Papunen L, Koivunen R, Perheentupa A, Ruokonen A, Tapanainen JS. Serum anti-Mullerian hormone levels remain high until late reproductive age and decrease during metformin therapy in women with polycystic ovary syndrome. Hum Reprod 2005;20:1820–6.
Medline Crossref
Rajpert-De Meyts E, Jorgensen N, Graem N, Muller J, Cate RL, Skakkebaek NE. Expression of anti-Mullerian hormone during normal and pathological gonadal development: association with differentiation of Sertoli and granulosa cells. J Clin Endocrinol Metab 1999;84:3836–44.
Medline Crossref
Rey R, Lukas C, Lasala C, Bedecarras P. AMH/MIS: what we know already about the gene, the protein and its regulation. Mol Cell Endocrinol 2003;211:21–31.
Medline Crossref
Silberstein T, MacLaughlin DT, Shai I, Trimarchi JR, Lambert G, Seifer DB, Keefe DL, Blazar AS. Mullerian inhibiting substance levels at the time of HCG administration in IVF cycles predict both ovarian reserve and embryo morphology. Hum Reprod 2006;21:159–63.
Medline Crossref
Smeenk JM, Sweep FC, Zielhuis GA, Kremer JA, Thomas CM, Braat DD. Antimullerian hormone predicts ovarian responsiveness, but not embryo quality or pregnancy, after in vitro fertilization or intracytoplasmic sperm injection. Fertil Steril 2007;87:223–6.
Medline Crossref
Soares SR, Simon C, Remohí J, Pellicer A. Cigarette smoking affects uterine receptiveness. Hum Reprod 2007;22:543–7.
Medline Crossref
Thomas JD, Rubin DN. Tissue harmonic imaging: why does it work? J Am Soc Echocardiogr 1998;11:803–8.
Medline
Tremellen KP, Kolo M, Gilmore A, Lekamge DN. Anti-Mullerian hormone as a marker of ovarian reserve. Aust NZ J Obstet Gynaecol 2005;45:20–4.
Medline Crossref
Veeck LL, ed. Atlas of human gametes and conceptuses. New York: Parthenon, 1999.
Vigier B, Picard JY, Tran D, Legeai L, Josso N. Production of anti-Mullerian hormone: another homology between Sertoli and granulosa cells. Endocrinology 1984;114:1315–20.
Medline Crossref
Waylen AL, Metwally M, Jones GL, Wilkinson AJ, Ledger WL. Effects of cigarette smoking upon clinical outcomes of assisted reproduction: a meta-analysis. Hum Reprod Update 2009;15:31–44.
Medline Crossref
Weenen C, Laven JS, Von Bergh AR, Cranfield M, Groome NP, Visser JA, Kramer P, Fauser BC, Themmen AP. Anti-Mullerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod 2004;10:77–83.
Medline Crossref
Weigert M, Hofstetter G, Kaipl D, Gottlich H, Krischker U, Bichler K, Poehl M, Feichtinger W.. The effect of smoking on oocyte quality and hormonal parameters of patients undergoing in vitro fertilization-embryo transfer. J Assist Reprod Genet 1999;16:287–93.
Medline Crossref