JBRA Assist. Reprod. 2016;20 (3):142-149
REVIEW ARTICLE
doi: 10.5935/1518-0557.20160032
Are there optimal numbers of oocytes, spermatozoa and embryos in assisted reproduction?
1SAGBAL Dr. Shterev, IVF Unit, Sofia, Bulgaria
CONFLICT OF INTERESTS
No conflict of interest have been declared.
ABSTRACT
The aim of this overview is to discuss the current information about
the search for the optimum yield of gametes in assisted reproduction,
as one of the major pillars of IVF success. The first topic is focused
on the number of male gametes and the possible impact of some genetic
traits on these parameters. The number of spermatozoa did not seem to
be crucial when there is no severe male factor of infertility. Genetic
testing prior to using those sperm cells is very important. Different
methods were applied in order to elect the “best” spermatozoa according
to specific indications. The next problem discussed is the importance
of the number of oocytes collected. Several studies have agreed that
“15 oocytes is the perfect number,” as the number of mature oocytes is
more important. However, if elective single embryo transfer is
performed, the optimal number of oocytes will enable a proper embryo
selection. The third problem discussed concerns fertility preservation.
Many educational programs promote and encourage procreation at maternal
ages between 20–35 years, since assisted reproduction is unable to
fully overcome the effects of female aging and fertility loss after
that age. It is also strongly recommended to ensure a reasonable number
of cryopreserved mature oocytes, preferably in younger ages (<35),
for which an average of two stimulation cycles are likely required. For
embryo cryopreservation, the “freeze all” strategy suggests the
vitrification of good embryos, therefore quality is prior to number and
patient recruitment for this strategy should be performed cautiously.
Keywords: Oocyte number, Mature oocyte, Spermatozoa count, Assisted reproduction, Fertility preservation.
INTRODUCTION
This review paper discusses current literature publications regarding
whether there is a specific mean number of oocytes, spermatozoa or
embryos giving an optimal chance for pregnancy outcome after assisted
reproduction, and whether the different methods chosen had benefits
regarding better outcomes.
1. Male gametes
1.1. Sperm number
With the development of intracytoplasmic sperm injection (ICSI) (Palermo et al., 1992; Van Steirteghem et al., 1993a, b) and testicular sperm extraction (TESE) (Devroey et al., 1994; 1996; Tournaye et al., 1996), and with TESE-ICSI (Tournaye et al., 1997, Tesarik et al., 1994; Tesarik & Sousa 1995),
the fathering possibility for patients with severe male factor
infertility became a reality. Pregnancy could be even achieved by ICSI,
even with totally immotile spermatozoa from the ejaculate or after
electroejaculation in men with spinal –cord injury (Barros et al., 1997; 1998).
One of the first large series reporting ICSI outcomes came from
Steirteghen (consisted of 1409 mature oocytes). They successfully
fertilized 64.2% of the oocytes (Van Steirteghem et al., 1993b).
A total of 67 pregnancies were achieved, of which 53 were clinical
(pregnancy rate of 44.7% per started cycle and 49.6% per embryo
transfer). The fertilization rate in this study was not influenced by
the standard semen concentration characteristics. ICSI offers
fertilization and pregnancy rates comparable to that achieved with
normal sperm count (WHO, 1999) for couples who
failed to achieve fertilization on repeated IVF cycles or had
spermatogenesis disorders (surgically retrieved or by
electroejaculation). Nevertheless, men with lower semen concentration
[<5mln/ml] had an increased risk for aneuploidy in the resulting
embryos (Campos-Galindo et al., 2015).
Severe oligozoospermia was also associated with higher percentage of
chromosomally abnormal spermatozoa (<1mln/ml: 31.4%) compared with
good semen counts (>80mln/ml: 6.5%) (Rodrigo et al., 2014). Whether the morphology of human sperm is also important is the matter of the following discussion.
1.2. Sperm selection
Sperm morphology evaluation, as a routine diagnostic tool, seemed to be
a powerful predictor of the fertilization potential of human
spermatozoa under in-vitro and in-vivo conditions (Kruger et al., 1986; 1988), and most of the sperm defects were significantly more frequent in infertile than in fertile men (Sa et al., 2015; Auger et al., 2016). Twelve years after the introduction of intracytoplasmic morphologically selected sperm injection (IMSI) (Setti et al., 2013)
procedure, based on the examination of motile sperm organelle
morphology (MSOME), seemed to be effective in overcoming the late
paternal effect and it is a promising real-time method for observation
and selection of motile and morphologically normal spermatozoa (Figure
1) for intracytoplasmic injection (ICSI) (Vanderzwalmen et al., 2008; Cassuto et al., 2012).
Also the fertilization of oocytes and development of embryos with high
implantation potential depends on the level of sperm DNA integrity (Franco et al., 2012) and oocyte activation (Sousa & Tesarik, 1994).
Several studies discovered that impairment of the morphological
characteristics in sperm heads (big vacuoles above 50% of the head
volume) had higher DNA fragmentation and aneuploidy rates compared with
spermatozoa with normal heads (Pedrix et al., 2011).
It was also suggested that sperm heads with big vacuoles could have
detrimental effects in early embryo development (Vanderzvalmen et al., 2008; Cassuto et al., 2012) and negative association with fertility potential (Bartoov et al., 1994; Mundy et al., 1994).
In order to decrease the miscarriage rate in couples with several
attempts a study recommended the preselection of spermatozoa with lack
of or with very small vacuoles (Berkovitz et al., 2006).
Also the IMSI leaded not only to higher percentage but also to improved
quality of resulting blastocysts on day 5 (Vanderzvalmen et al., 2008; Cassuto et al., 2012; Knez et al., 2011), and had better implantation and pregnancy rates (Berkovitz et al., 2006). According to some authors, severe teratozoospermia was in correlation with lower fertilization and fragility of sperm DNA (Marci et al., 2013). The impact of IMSI on good embryo quality at day 3 and their availability for cryopreservation was also suggested recently (Dyulgerova-Nikolova et al., 2015).
Despite those studies, some authors found that IMSI did not improve the
outcome of patients with two successive IVF-ICSI failures (Gatimel et al., 2016),
and more studies especially focused on different types of male
infertility and the impact of IMSI procedure need to be performed. But
as Vanderzwalmen & Fallet (2010) proposed: “Are there any indications to not select the best spermatozoa? Of course not.”(Setti et al., 2013).
Some other techniques, such as the selection of the fast motile
spermatozoa during ICSI, may improve the qualities of the fertilizing
spermatozoon by decreasing aneuploidy rates for chromosomes X, Y and 18
among preselected ones in men with severe teratozoospermia (Levron et al., 2013).
Magnetic-Activated Cell Sorting (MACS) is also a promising method for
obtaining sperm cells with non-apoptotic DNA material and, therefore,
higher capability for fertilization and producing viable good quality
embryos (Bucar et al., 2015). The method
is based on the binding of superparamagnetic Annexin-microbeads to
externalized phosphatidylserine (PS) at the outer leaflet of the
sperm’s plasma membrane with activated apoptosis signaling, or membrane
damage (Grunewald & Paasch, 2013). The
efficiency is still questionable and there is not enough information
about the benefits of it in order to replace traditional sperm
selection. Nevertheless, MACS could be useful in cases where poor sperm
quality (oligo/astheno-zoospermia) and recurrent implantation failures
were seen (Bochev et al., 2011) and thus, lead to improved clinical pregnancy rates (Bucar et al., 2015).
Favoring selection of spermatozoa with intact DNA and normal nucleus,
hyaluronan assay (HA) may be another alternative to optimize ICSI
outcomes. In fact, some studies have shown significant differences (Parmegiani et al., 2010), while others did not find differences in clinical pregnancy rates or embryo quality (Majumdar & Majumdar, 2013). There is also a reduced pregnancy loss when combinations of both methods were applied (Paasch et al., 2007).
Birefringence still has some contribution in male infertility diagnosis
and treatment. Some authors claimed that sperm head’s birefringence
could be used as a new criterion for sperm selection (Gianaroli et al., 2008).
Although fertilization and cleavage rates did not differ between the
study and control groups, in the most severe male factor condition, the
rates of clinical pregnancy, ongoing pregnancy, and implantation were
significantly higher.
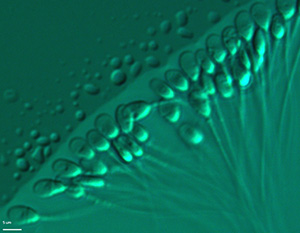
Figure 1. Selection after evaluation of motile sperm organelle morphology (MSOME) at x6000 magnification prior to ICSI.
1.3. Impact of genetic changes in sperm
Currently, genetic testing is indicated primarily to elucidate the
underlying diagnosis and to assess the risk to the offspring following
successful treatment by e.g. ICSI, TESE etc. According to international
guidelines, pre- and post-genetic test counselling by an appropriately
trained professional is highly desirable, and in some countries even
mandatory (Harper et al., 2014). There were also some major regulatory mechanisms that had gene defects associated (Pereira et al., 2015),
with specific morphological and motility abnormalities. An “indication
threshold” for genetic testing is the total sperm count concentration
below 10 million per milliliter (<10x106/ml). Recently, it has been
estimated that about 25% of patients with azoospermia and severe
oligozoospermia should undergo genetic testing. Molecular cytogenetics
proved that lower sperm count was associated with higher level of
autosomal and gonosomal aberrations (mainly represented in Klinefelter
syndrome: 47, XXY, including various mosaics), that could be passed on
to the next generation. Healthy children were born even from sperm from
those men after adequate diagnosis and treatment (Madureira et al., 2014).
Unfortunately, most of the cases have been first diagnosed with lower
sperm count and male infertility and then the men underwent genetic
testing.
The majority of men with chromosomal aberrations
associated with infertility are apparently healthy males but with
various forms of chromosomal translocations. They have higher risk of
causing repeated miscarriages or stillbirth in their offspring, since
their balanced translocations commonly get “unstable” by producing a
variety of abnormal gametes (Alves et al., 2002a; Harper et al., 2014).
Interestingly, some authors found that globozoospermia could be considered as “a new genomic disorder” (Elinati et al., 2012).
The study confirmed that DPY19L2 was the major gene responsible for
globozoospermia. To date, mutations in two genes, SPATA16 and DPY19L2,
have been identified as responsible for this severe teratozoospermia.
In addition, approximately 1% of all infertile men were born with the
congenital absence of vas deferens (CBAVD). Pathogenic mutations in the
cystic fibrosis transmembrane receptor gene (CFTR) are associated with
obstructive azoospermia due to CBAVD and, therefore, those patients are
candidates for CFTR testing.
Various Y-chromosome deletions are predominantly found in
non-obstructive azoospermia or severe oligozoospermia, and Sertoli cell
only syndrome (Ferras et al., 2004; Fernandes et al., 2002; Kamp et al., 2001).
Association of AZFa, AZFb, AZFbc and AZFc microdeletions with
infertility is unambiguous. The majority of these deletions are in the
AZFc region. Azoospermic men have a higher prevalence of microdeletions
than oligozoospermic patients (Ferrás et al., 2004; Fernandes et al., 2006; Gonçalves et al., 2016).
The data showed that no sperm could be retrieved in AZFa, AZFb, while
there is a lower chance that viable sperm could be retrieved from AZFc
cases.
In conclusion, genetic counselling should be offered to the
family, as well as PGD or PGS, as a part of fertility treatment, since
the underlying cause of male infertility is transferred to successive
generations (Alves et al., 2002b; Pinho et al., 2005; Harper et al., 2014).
Future prospective studies for genetic treatment of male could be
through flow cytometry cell sorting (FCCS) for separation of sperm
cells (Vidal et al., 1998). This method is applied mainly for prevention of sex-linked genetic disorders (Dondorp et al., 2013).
The method is based on staining of spermatozoa with specific markers
and sorting them with flow cytometry, which provides a means of
preventing significant disease in the offspring, and may help reduce
the number of supernumerary affected embryos prior to preimplantation
genetic diagnosis (De Geyter et al., 2013).
2. Female gametes
2.1. Oocyte number
Because of the limited number of oocytes available after ovarian
stimulation, their count and quality seem to be of a great importance
for advanced maternal age (AMA) and/or premature ovarian insufficiency
(POI), with dramatically decrease of the number and implantation
potential of oocytes together with a dramatic pick up of aneuploidy
rates over 70% (NIHCE, 2013).
IN addition, women with AMA have higher risk for miscarriages (over
40%) as well as drastically lower rates of clinical pregnancies.
If a natural cycle takes place in the beginning of IVF (Steptoe & Edwards, 1978), the likelihood of young women (<35) taking a baby home is only 3.8%; 1.3% (40-42 age) and 0% (>43 age) (HFEA, 2015).
The live birth rates in natural cycle in poor responders and poor
ovarian insufficiency (POR, POI) patients according to the Bologna
criteria are significantly lower (2.6%) (Polyzos et al., 2012).
These findings can be explained by the “pregnancy loss iceberg” model,
in which 60% of all obtained embryos are lost in pre/post implantation
period and additionally, 15% of them drop out due to miscarriage, where
over 40% are due to chromosomal aneuploidy (Azmanov et al., 2007). Some authors even called this iceberg as “the black box of early pregnancy loss” (Macklon et al., 2002).
Thus, in the last decade, different protocols for controlled ovarian
hyperstimulation (COH) have been modified in order to optimize the
number, quality and maturity of oocytes. Systematic reviews and
meta-analyses have been published in order to evaluate stimulation
duration, number of oocytes per egg retrieval and ongoing pregnancy
rates (Bodri et al., 2011; Stimpfel et al., 2015), and the risk of ovarian hyperstimulation syndrome (OHSS) (Sousa et al., 2015).
Several studies released by the European Society of Human Reproduction
and Embryology (ESHRE) summarized that “15 oocytes is the perfect
number” and suggested an optimal chance for achieving a pregnancy in
one cycle (Sunkara et al., 2011),
with the percentage going up to 37% when 15 oocytes had been collected.
According to a large cohort study, the oocyte count is not as important
as the optimal number of mature oocytes in metaphase 2 (mean number 9
and more metaphase 2) are collected (Bals-Pratsch et al., 2010).
In cases of more than 16 oocytes retrieved, the pregnancy rate
decreases slowly, but the risk for hyperstimulation increases
drastically. Other authors found that the pregnancy rate per embryo
transfer reached the absolute maximum (30.8%) when between 11 and 15
mature oocytes were available for ICSI injection (Steward et al., 2014)
in a single embryo transfer (SET) strategy. Among the studies, there is
another suggestion for so called “gold standard” with variation between
5-15 oocytes (Timeva et al., 2006). A
different mean number of oocytes per collection have been proposed as
optimal, but all studies agree that patient safety and health are the
most important and the risk of OHSS should be evaluated carefully and
minimized (Sousa et al., 2015).
2.2. Oocyte morphology
Different types of dimorphisms in cumulus-oocyte complexes and in
oocyte morphology (ooplasm, perivitelline space, first polar body and
zona pellucida, giant oocytes) have been evaluated over the years (Mandelbaum, 2000),
especially when the ICSI method was performed. This technique has
enabled the precise assessment of oocyte morphology. There are many
conflicting results in the literature concerning the incidence of
oocyte dimorphism on fertilization rates, or potential further
implantation and development. Most of the studies found an increased
incidence of aneuploidy among dysmorphic oocytes (Van Blerkom & Henry, 1992; Kahraman et al., 2000).
Different equipment and software have been developed
(birefringence/polarization microscopy) in order to evaluate the
spindle position of mature oocytes (Figure 2). Some studies are focused
on birefringent spindles and the prediction of fertilization rates
after intracytoplasmic sperm injection (ICSI). The results indicated
that the presence of a spindle in human oocytes can predict not only
higher fertilization rates, but also higher embryo developmental
competence (Wang et al., 2001). One study (Montag et al., 2011)
criticizes the majority, claiming that they are only observational and
not performed in a randomized manner, using other gamete selection
markers for comparison. Despite that, polarization microscopy may help
improve knowledge on meiosis. Whether or not certain applications such
as spindle or zone imaging may lead to an increase in IVF success
presently remains unclear.
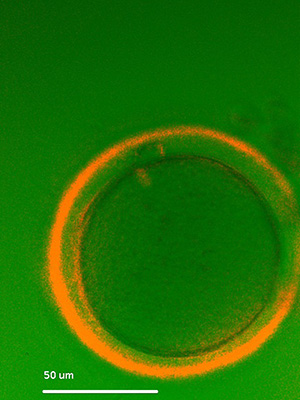
Figure 2. Evaluation of spindle position and zona pellucida birefringence of an oocyte at x300 magnification prior to ICSI.
3. Human preimplantation embryo genetics
The probability of obtaining optimal embryo quality and to have spare
embryos for cryopreservation (CP) depends on oocyte and sperm count and
quality. The chance for having a good blastocyst from one cohort of
gametes increases if some other additional methods (e.g. MSOME) are
applied.
Since its first application by Handyside et al. (1990)
for an X-linked disorder in more than 1,000 healthy children born after
preimplantation genetic diagnosis (PGD) and annual screening (PGS) (De Rycke et al., 2015).
Sex-linked disorders, monogenic diseases, chromosomal abnormalities and
translocations in either or both partners are some of the major
indications.
The most challenging part of the PGD combined with HLA
typing is the low probability of transfer in these cycles. Genetic
counselors should provide the patient with the information that there
is only 18.7% chance of finding an HLA-identical and healthy embryo,
such as for beta-thalassemia (Milachich et al., 2013).
Therefore, considerable numbers of oocytes and embryos are required in
order to select disease-free and HLA-compatible embryos for transfer.
Blastocoel fluid and DNA extraction is the first non-biopsy method
which was suggested for PGD or PGS purposes. Genomic DNA in human
blastocoel fluid was already defined by the teams of Palini et al. (2013) and Gianaroli et al. (2014) although there are still some questions about representability of obtained results (Cohen et al., 2013).
Probably, the non-invasive PGS will spread in the future, together with
non-invasive prenatal diagnosis and testing (NIPD, NIPT) (Milachich, 2014).
4. Human embryo morphokinetics
The application of embryo time–lapse imaging
(Figure 3) could be used as a predictor for good implantation and lower
aneuploidy rate among transferable embryos. Much discussed studies (Meseguer et al., 2011; Campbell et al., 2013)
reported that morphokinetics could be associated with the aneuploidy
incidence. Embryo aneuploidy, a major cause of IVF failure, has been
correlated with specific morphokinectic variables used previously to
develop an aneuploidy risk classification model. The study by Campbell et al., 2013,
evaluated the effectiveness and potential impact of this model for
unselected IVF patients without biopsy and preimplantation genetic
screening (PGS), and discovered a significant difference in LBR between
embryos classified with low, medium and high risk by demonstrating the
clinical relevance of the novel aneuploidy risk classification model.
There was a cautionary note against time-lapse imaging (Ottolini et al., 2014) and its inability to establish embryo aneuploidy risk.
A recent randomized controlled trial by Goodman et al. (2016)
stated that the use of time-lapse morphokinectic data did not
significantly improve clinical reproduction outcomes in all patients
and in those with blastocyst transfers. Also, the findings from other
systematic reviews did not support the routine use of time-lapse in
clinical IVF. Therefore, future studies evaluating this technology in
well-designed trials should be performed (Racowsky et al., 2015).
Although the use of time-lapse by itself is controversial and
debatable, more and more studies are in favor of using this system for
the selection of the most viable embryo to transfer, minimizing the
early pregnancy loss from 25.8% to 16.6% (Campbell et al., 2013).
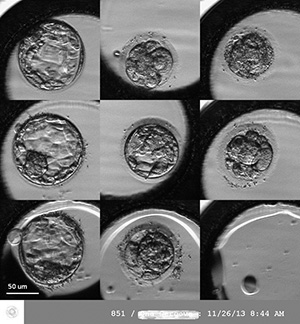
Figure 3. Time-lapse imaging of human embryos on day 5 (120 hours after in vitro fertilization).
5. Cryopreservation – count of oocytes and embryos
Major advances achieved in the past few years in the cryo-laboratory
(e.g. vitrification) have brought about significant changes to the
practice. Oocyte fertility preservation (OFP) for social or oncologic
reasons, the possibility to create oocyte banks for egg donation
programs, the opportunity to avoid ovarian hyperstimulation syndrome
(“freeze all” embryos) or a strategy to accumulate oocytes in low-yield
patients and then transfer embryos in a natural cycle are some of the
options that are now available with the development of cryopreservation.
Each year of maternal age increase, decreases the delivery rate by 7%.
Survival rate and female age reflect oocyte quality, which declines
with age. Women at age 40 face a 40% chance of miscarriage if they can
get pregnant at all, and by the age of 45, the risk of miscarriage is
75%. Freezing a woman’s eggs around the age of 30 “freezes the time”
her fertility potential, and gives her the chance of a healthy
pregnancy at a time of her choosing (Lockwood et al., 2011), but the role of oocyte cryopreservation in a real chance for obtaining pregnancy is still debatable.
Vitrification of oocytes from fertile young patients can produce high
pregnancy rates. The inclusion criteria for OFP are: age (<35) at
the time of recruitment; prior tubal ligation after the last child;
body mass index <30 and basal antral follicle count >10. One of
the recently proposed strategies (Devine et al., 2015)
was to have patients undergo oocyte cryopreservation before the age of
35 years and to obtain at least 16 MII oocytes for potential use after
the age of 40 years. Within this strategy, women attempt spontaneous
conception by timed intercourse for a period of 6 months when reaching
age of 40 years. If no spontaneous pregnancy is obtained, the women are
then submitted to two IVF cycles using previously banked oocytes.
Another multicentric prospective cohort study reported no differences
concerning survival, fertilization, and embryo development, but with
three determinants of success: patient age (<38 years), number of
vitrified MII oocytes (≥8), and blastocyst stage on embryo transfer.
Additionally, when the number of vitrified oocytes was higher, delivery
rates increased from 22.6% to 46.4% (Rienzi et al., 2012). There is another study with comparable results where the mean number of vitrified oocytes was 7.2 (Cobo et al., 2012a).
Other studies found that pregnancy rates per thawed oocyte varied
between 4,5% and 15%, where most women were younger than 35 years. The
overall pregnancy rate ranged between 36-61% (Practice Committees of American Society for Reproductive Medicine & Society for Assisted Reproductive Technology, 2013), but again the female age was strongly related with a higher chance for extending reproductive options (Cobo & Garcia-Velasco, 2016).
A recent study found that the oocyte vitrification for elective
fertility preservation was an efficient option when at least 8 to 10
metaphase II oocytes were collected and, thus a reasonable success was
achieved (Cobo et al., 2016). Also, the
live birth rate was correlated with the additive role of every
collected oocyte (gain of 8.4% per additional oocyte) in the group of
women below 35 years of age.
Unfortunately, very few studies
reported clinical outcomes with vitrified oocytes in cancer patients.
Progress in cancer treatment using radiotherapy or chemotherapy has
improved survival rates among malignant diseases. This is particularly
evident in children and breast cancer patients. For most oncologic
patients there is a chronic adverse effect of radiation or cytotoxic
chemotherapy, including gonadal failure and infertility, which often
cause distress, low self-esteem and undermined quality of life. Thus,
the need is evident for an effective OFP strategy that provides the
chance to conceive a child with one’s own gametes. Special care must be
paid to any condition and to the decision on the number of oocytes to
be stored. Patients must be counseled objectively, according to their
possibilities and current evidence to avoid false hopes, especially in
cancer patients, where interdisciplinary collaboration with
oncologists, psychologists and gynecologists is required (Waldby, 2015).
Since AR is unable to fully overcome the effects of age on fertility
loss after the age of 35 years and additionally, a higher proportion of
maternal and/or fetal morbidity and mortality are associated with
advanced maternal age. Therefore, many medical organizations promote
educational programs that encourage procreation or egg freezing at a
maternal age of 20–35 years. It was strongly suggested to advise and to
inform patients that they should ensure a reasonable number of
cryopreserved oocytes, for which more than one stimulation cycle is
likely required. To date, there is good evidence to disregard oocyte
vitrification and experimental warming since it had similar pregnancy
rates as the use of fresh oocytes for IVF/ICSI procedure in the group
of young patients (Practice Committees of American Society for Reproductive Medicine & Society for Assisted Reproductive Technology, 2013) for fertility preservation purposes.
Another recent strategy for improving IVF outcomes is the “freeze-all”
embryo policy. Even in a group of patients that was selected for fresh
ET (P≤1.5
ng/mL), implantation may be impaired by COH, and outcomes may be
improved. COH may contribute to endometrium modifications, which might
be related to poorer outcomes when fresh ET is performed. In cycles
with fresh ET, there is still a risk of OHSS. Thus, this strategy was
implemented for special cases: in which P was >1.5 ng/mL
on the trigger day; women aged 20–45 years; fresh and frozen-thawed ET
performed with good-quality embryos only (Roque, 2015).
Some exclusion criteria were: patients with a history of recurrent
pregnancy loss; implantation failure (≥3 previous attempts); antral
follicle count ≤5; severe male factor infertility (oligospermia <1
million/mL, and azoospermia) (WHO, 1999); uterine
pathology. Clinical pregnancy rate was lower (35,9%) in the fresh cycle
group, whereas when the freeze all strategy was applied this rate went
up (46.4%).
Another study (Cobo et al., 2012b)
included a large cohort of patients (3,150 warming cycles), where the
policy for embryo cryopreservation depended on the morphologic quality
and only optimum and good quality embryos were cryopreserved in women
with risk of OHSS, impaired endometrium pattern, or high progesterone
levels. Thus the clinical pregnancy rate of vitrified day 5 embryo
varies between 41.7% and 49.3% per transfer, respectively, according to
their “optimal” or “very good” quality.
Further randomized clinical
trials are needed to confirm the advantage of this strategy and
determine for which group of patients the “freeze all strategy” would
be most beneficial.
CONCLUSION
In conclusion, the
mature oocyte count, with maternal age and the proper sperm selection
might be the major or dominate circumstances for obtaining better
outcome in IVF/ICSI cycles, SET cycles, embryo cryopreservation and
oocyte fertility preservation, but still further trials are needed in
order to evaluate the role of each one of these factors.
Acknowledgements
To the
scientific adviser of this review article Prof. St.Kyurkchiev, MD, PhD,
Institute of Reproductive Health, Sofia, for the intellectual support
given to this paper.
REFERENCES
Alves
C, Carvalho F, Cremades N, Sousa M, Barros A. Unique (Y;13)
translocation in a male with oligozoospermia. Cytogenetic and molecular
studies. Eur J Hum Genet. 2002a; 10: 467-74.
Medline Crossref
Alves
C, Sousa M, Silva J, Barros A. Preimplantation genetic diagnosis using
FISH for carriers of robertsonean translocations: the portuguese
experience. Prenatal Diagnosis. 2002b; 22:1153-62.
Medline Crossref
Auger J, Jouannet P, Eustache F. Another look at human sperm morphology. Hum Reprod 2016; 31:10-23.
Medline Crossref
Azmanov
D, Milachich T, Zaharieva B, Michailova GI, Dimitrova VG, Karagiozova
ZH, Maznejkova VT, Chernev TA, Toncheva DI. Profile of chromosomal
aberrations in different gestational age spontaneous abortions detected
by comparative genomic hybridization. Eur J Obstet Gynecol Reprod Biol.
2007;131:127-31.
Medline Crossref
Bals-Pratsch
M, Bühler K, Krüssel J, Wendelken M Dahncke W, Kupka MS. Extended
Analyses of the German IVF Registry (DIR): Andrological Aspects,
Medical-Economical Assumptions Related to the Shift from IVF to ICSI
and Stimulation with Gonadotropins J. Reproduktionsmed Endokrinol.
2010; 7:40-4.
Link
Barros
A, Sousa M, Oliveira C, Silva J, Almeida V, Beires J. Pregnancy and
birth after intracytoplasmic sperm injection with totally immotile
sperm from the ejaculate. Fertil Steril. 1997;67:1091-4.
Medline Crossref
Barros
A, Sousa M, Andrade MJ, Oliveira C, Silva J, Beires J. Birth after
electroejaculation coupled to intracytoplasmic sperm injection in a
gun-shot spinal-cord injured man. Arch Androl. 1998;41:5-9.
Medline Crossref
Bartoov
B, Eltes F, Pansky M, Langzam J, Reichart M, Soffer Y. Improved
diagnosis of male fertility potential via a combination of quantitative
ultramorphology and routine semen analysis. Hum Reprod. 1994; 9:
2069-75.
Medline
Berkovitz
A, Eltes F, Ellenbogen A, Peer S, Feldberg D, Bartoov B. Does the
presence of nuclear vacuoles in human sperm selected for ICSI affect
pregnancy outcome? Hum Reprod. 2006; 21:1787-90.
Medline Crossref
Bochev
I, Gavrilov P, Kurkchiev S, Shterev A. Sperm motility improvement after
magnetic-activated cell sorting in men with
astheno/oligo-asthenozoospermia. Hum Reprod. 2011;26:i133.
Crossref
Bodri
D, Sunkara SK, Coomarasamy A. Gonadotropin-releasing hormone agonists
versus antagonists for controlled ovarian hyperstimulation in oocyte
donors: a systematic review and meta-analysis. Fertil Steril. 2011;
95:164-9.
Medline Crossref
Bucar
S, Gonçalves A, Rocha E, Barros A, Sousa M, Sá R. DNA fragmentation in
human sperm after magnetic-activated cell sorting. J Assist Reprod
Genet. 2015;32:147-54.
Medline Crossref
Campbell
A, Fishel S, Bowman N, Duffy S, Sedler M, Hickman C. Modelling a risk
classification of aneuploidy in human embryos using non-invasive
morphokinetics. Reprod Biomed Online. 2013; 26:477-85.
Medline Crossref
Campos-Galindo
I, García-Herrero S, Martínez-Conejero JA, Ferro J, Simón C, Rubio C.
Molecular analysis of products of conception obtained by
hysteroembryoscopy from infertile couples. J Assist Reprod Genet.
2015;32:839-48.
Medline Crossref
Cassuto
N, Hazout A, Hammoud I, Balet R, Bouret D, Barak Y, Jellad S, Plouchart
JM, Selva J, Yazbeck C. Correlation between DNA defect and sperm-head
morphology. Reprod Biomed Online. 2012; 24:211-8.
Medline Crossref
Cobo
A, Garrido N, Crespo J, José R, Pellicer A. Accumulation of oocytes: a
new strategy for managing low-responder patients. Reprod Biomed Online.
2012a; 24:424-32.
Medline Crossref
Cobo
A, de los Santos, MJ, Castellò D, Gámiz P, Campos P, Remohí J. Outcomes
of vitrified early cleavage-stage and blastocyst-stage embryos in a
cryopreservation program: evaluation of 3,150 warming cycles. Fertil
Steril. 2012b;98:1138–46.
Medline Crossref
Cobo
A, García-Velasco JA, Coello A, Domingo J, Pellicer A, Remohí J. Oocyte
vitrification as an efficient option for elective fertility
preservation. Fertil Steril. 2016;105:755-64.
Medline Crossref
Cobo A, García-Velasco JA. Why all women should freeze their eggs. Curr Opin Obstet Gynecol. 2016; 28:206-10.
Medline Crossref
Cohen
J, Grudzinskas G, Johnson M. Embryonic DNA sampling without biopsy: the
beginnings of non-invasive PGD? Reprod BioMed Online. 2013:26;520-1.
Medline Crossref
De
Geyter C, Sterthaus O, Miny P, Wenzel F, Lapaire O, De Geyter M,
Sartorius G. First successful pregnancy in Switzerland after
prospective sex determination of the embryo through the separation of
X-chromosome bearing spermatozoa. Swiss Med Wkly. 2013; 143:w13718.
Medline Crossref
De
Rycke M, Belva F, Goossens V, Moutou C, SenGupta SB, Traeger-Synodinos
J, Coonen E. ESHRE PGD Consortium data collection XIII: cycles from
January to December 2010 with pregnancy follow-up to October 2011. Hum
Reprod. 2015; 30:1763-89.
Medline Crossref
Devine
K, Mumford SL, Goldman KN, Hodes-Wertz B, Druckenmiller S, Propst AM,
Noyes N. Baby budgeting: oocyte cryopreservation in women delaying
reproduction can reduce cost per live birth. Fertil Steril.
2015;103:1446-53.
Medline Crossref
Devroey
P, Liu J, Nagy Z, Tournaye H, Silber SJ, Van Steirteghem AC. Normal
fertilization of human oocytes after testicular sperm extraction and
ICSI. Fertil Steril. 1994; 62:639-41.
Medline
Devroey
P, Nagy P, Tournaye H, Liu J, Silber S, Van Steirteghem A. Outcome of
intracytoplasmic sperm injection with testicular spermatozoa in
obstructive and non-obstructive azoospermia. Hum Reprod. 1996;11:1015-8.
Medline
Dondorp
W, De Wert G, Pennings G, Shenfield F, Devroey P, Tarlatzis B, Barri P,
Diedrich K. ESHRE Task Force on ethics and Law 20: sex selection for
non-medical reasons. Hum Reprod. 2013;28:1448-54.
Medline Crossref
Elinati
E, Kuentz P, Redin C, Jaber S, Vanden Meerschaut F, Makarian J,
Koscinski I, Nasr-Esfahani MH, Demirol A, Gurgan T, Louanjli N, Iqbal
N, Bisharah M, Pigeon FC, Gourabi H, De Briel D, Brugnon F, Gitlin SA,
Grillo JM, Ghaedi K, Deemeh MR, Tanhaei S, Modarres P, Heindryckx B,
Benkhalifa M, Nikiforaki D, Oehninger SC, De Sutter P, Muller J,
Viville S. Globozoospermia is mainly due to DPY19L2 deletion via
non-allelic homologous recombination involving two recombination
hotspots. Hum Mol Genet. 2012; 21:3695-702.
Medline Crossref
Fernandes
AT, Fernandes S, Gonçalves R, Sá R, Costa P, Rosa A, Ferrás C, Sousa M,
Brehm A, Barros A. DAZ gene copies: evidence of Y chromosome evolution.
Mol Hum Reprod. 2006;12: 519-23.
Medline Crossref
Fernandes
S, Huellen K, Gonçalves J, Dukal H, Zeisler J, de Meyts ER, Skakkebaek
NE, Habermann B, Krause W, Sousa M, Barros A, Vogt PH. High frequency
of DAZ1/DAZ2 gene deletions in patients with severe oligozoospermia.
Mol Hum Reprod. 2002;8:286-98.
Medline Crossref
Ferrás
C, Fernandes S, Marques CJ, Carvalho F, Alves C, Silva J, Sousa M,
Barros A. AZF and DAZ gene copy specific deletion analysis in
maturation arrest and Sertoli cell only syndrome. Mol Hum Reprod.
2004;10:755-61.
Medline Crossref
Franco
JG Jr, Mauri AL, Petersen CG, Massaro FC, Silva LF, Felipe V, Cavagna
M, Pontes A, Baruffi RL, Oliveira JB, Vagnini LD. Large nuclear
vacuoles are indicative of abnormal chromatin packaging in human
spermatozoa. Int J Androl. 2012; 35:46-51.
Medline Crossref
Gatimel
N, Parinaud J, Leandri RD. Intracytoplasmic morphologically selected
sperm injection (IMSI) does not improve outcome in patients with two
successive IVF-ICSI failures. J Assist Reprod Genet. 2016; 33:349-55.
Medline Crossref
Gianaroli
L, Magli MC, Pomante A, Crivello AM, Cafueri G, Valerio M, Ferraretti
AP. Blastocentesis: a source of DNA for preimplantation genetic
testing. Results from a pilot study. Fertil Steril. 2014;102:1692-9.
Medline Crossref
Gianaroli
L, Magli MC, Collodel G, Moretti E, Ferraretti AP, Baccetti B. Sperm
head’s birefringence: a new criterion for sperm selection. Fertil
Steril. 2008; 90:104-12.
Medline Crossref
Gonçalves
C, Cunha M, Rocha E, Fernandes S, Silva J, Ferraz L, Oliveira C, Barros
A, Sousa M. Y chromosome microdeletions in non-obstructive azoospermia
and severe oligozoospermia. Asian J Androl. 2016. In press.
Medline Crossref
Goodman
LR, Goldberg J, Falcone T, Austin C, Desai N. Does the addition of
time-lapse morphokinetics in the selection of embryos for transfer
improve pregnancy rates? A randomized controlled trial. Fertil Steril.
2016;105:275-85.
Medline Crossref
Grunewald S, Paasch U. Basic diagnostics in andrology. J Dtsch Dermatol Ges. 2013; 11:799-814.
Medline Crossref
Handyside
A, Kontogianni E, Hardy K, Winston RM. Pregnancies from biopsied human
preimplantation embryos sexed by Y specific DNA amplification. Nature.
1990; 344: 768–70.
Medline Crossref
Harper
J, Geraedts J, Borry P, Cornel MC, Dondorp WJ, Gianaroli L, Harton G,
Milachich T, Kääriäinen H, Liebaers I, Morris M, Sequeiros J, Sermon K,
Shenfield F, Skirton H, Soini S, Spits C, Veiga A, Vermeesch JR,
Viville S, de Wert G, Macek M Jr; ESHG, ESHRE and EuroGentest2 Current
issues in medically assisted reproduction and genetics in Europe:
research, clinical practice, ethics, legal issues and policy. Hum
Reprod. 2014; 29:1603-9.
Medline Crossref
HFEA -Human Fertilisation and Embryology Authority, UK. Natural cycle IVF. 2015. Available at: http://www.hfea.gov.uk/natural-cycle-ivf.html
Kahraman
S, Yakin K, Dönmez E, Samli H, Bahçe M, Cengiz G, Sertyel S, Samli M,
Imirzalioğlu N. Relationship between granular cytoplasm of oocytes and
pregnancy outcome following intracytoplasmic sperm injection. Hum
Reprod. 2000; 15:2390-3.
Medline Crossref
Kamp
YC, Huellen K, Fernandes S, Sousa M, Schlegel PN, Mielnik A, Kleiman S,
Yavetz H, Krause W, Kupker W, Johannisson R, Schulze W, Weidner W,
Barros A, Vogt PH. High deletion frequency of the complete AZFa
sequence occurs only in men with Sertoli-cell-only-syndrome. Mol Hum
Reprod. 2001;7:987-94.
Medline Crossref
Knez
K, Zorn B, Tomazevic T, Vrtacnik-Bokal E, Virant-Klun I. The IMSI
procedure improves poor embryo development in the same infertile
couples with poor semen quality: a comparative prospective randomized
study. Reprod Biol Endocrinol. 2011; 9:123.
Medline Crossref
Kruger
TF, Acosta AA, Simmons KF, Swanson RJ, Matta JF, Oehninger S.
Predictive value of abnormal sperm morphology in in vitro
fertilization. Fertil Steril. 1988; 49:112-7.
Medline
Kruger
TF, Menkveld R, Stander FS, Lombard CJ, Van der Merwe JP, van Zyl JA,
Smith K. Sperm morphologic features as a prognostic factor in in vitro
fertilization. Fertil Steril. 1986;46:1118-23.
Medline
Levron
J, Aviram-Goldring A, Rienstien S, Bider D, Dor J, Raviv G. Aneuploidy
rates for chromosomes X/Y and 18 among preselected spermatozoa in men
with severe teratospermia. Reprod Biomed Online. 2013; 27:280-5.
Medline Crossref
Lockwood
GM. Social egg freezing: the prospect of reproductive ‘immortality’ or
a dangerous delusion? Reprod Biomed Online. 2011; 23:334-40.
Medline Crossref
Macklon
NS, Geraedts JP, Fauser BC. Conception to ongoing pregnancy: the ‘black
box’ of early pregnancy loss. Hum Reprod Update. 2002; 8:333-43.
Medline Crossref
Madureira
C, Cunha M, Sousa M, Neto AP, Pinho MJ, Viana P, Gonçalves A, Silva J,
da Silva JT, Oliveira C, Ferraz L, Dória S, Carvalho F, Barros A.
Treatment of 128 azoospermic patients with non-mosaic Klinefelter
syndrome with birth of 17 healthy children. Andrology. 2014; 2:623-31.
Medline Crossref
Majumdar
G, Majumdar A. A prospective randomized study to evaluate the effect of
hyaluronic acid sperm selection on the intracytoplasmic sperm injection
outcome of patients with unexplained infertility having normal semen
parameters. J Assist Reprod Genet. 2013; 30:1471-5.
Medline Crossref
Mandelbaum J. Oocytes. Hum Reprod. 2000;15:11-8.
Medline Crossref
Marci
R, Murisier F, Lo Monte G, Soave I, Chanson A, Urner F, Germond M.
Clinical outcome after IMSI procedure in an unselected infertile
population: a pilot study. Reprod Health. 2013;10:16.
Medline Crossref
Meseguer
M, Herrero J, Tejera A, Hilligsøe K, Ramsing N, Remohí J. The use of
morphokinetics as a predictor of embryo implantation. Hum Reprod. 2011;
26:2658-71.
Medline Crossref
Milachich
T, Timeva T, Ekmekci C, Beyazyurek C, Tac HA, Shterev A, Kahraman S.
Birth of a healthy infant after preimplantation genetic diagnosis by
sequential blastomere and trophectoderm biopsy for ß-thalassemia and
HLA genotyping. Eur J Obstet Gynecol Reprod Biol. 2013;169:261–7.
Medline Crossref
Milachich
T. New advances of preimplantation and prenatal genetic screening and
noninvasive testing as a potential predictor of health status of
babies. Biomed Res Int. 2014; 306505.
Medline Crossref
Montag
M, Köster M, van der Ven K, van der Ven H. Gamete competence assessment
by polarizing optics in assisted reproduction. Hum Reprod Update.
2011;17:654-66.
Medline Crossref
Mundy
AJ, Ryder TA, Edmonds DK. A quantitative study of sperm head
ultrastructure in subfertile males with excess sperm precursors. Fertil
Steril. 1994;61:751-4.
Medline
NIHCE
- National Institute for Health and Clinical Excellence. Fertility:
Assessment and Treatment for People with Fertility Problems 2013
National Collaborating Centre for Women’s and Children’s Health (UK).
London: Royal College of Obstetricians & Gynaecologists, 2013.
Link
Ottolini
C, Rienzi L, Capalbo A A cautionary note against embryo aneuploidy risk
assessment using time-lapse imaging. Reprod Biomed Online.
2014;28:273-5.
Medline Crossref
Paasch U, Grunewald S, Glander HJ. Sperm selection in assisted reproductive techniques. Soc Reprod Fertil Suppl. 2007;65:515-25.
Medline
Palermo
G, Joris H, Devroey P, Van Steirteghem AC. Pregnancies after
intracytoplasmic injection of single spermatozoon into an oocyte.
Lancet. 1992;340:17–8.
Medline Crossref
Palini
S, Galluzzi L, De Stefani S, Bianchi M, Wells D, Magnani M, Bulletti C.
Genomic DNA in human blastocoele fluid. Reprod BioMed Online.
2013;26:603-10.
Medline Crossref
Parmegiani
L, Cognigni GE, Ciampaglia W, Pocognoli P, Marchi F, Filicori M.
Efficiency of hyaluronic acid (HA) sperm selection. J Assist Reprod
Genet. 2010; 27:13-6.
Medline Crossref
Pedrix
A, Travers A, Chelli MH, Escalier D, Do Rego JL, Milazzo JP,
Mousset-Siméon N, Macé B, Rives N. Assessment of acrosome and nuclear
abnormalities in human spermatozoa with large vacuoles. Hum Reprod.
2011; 26:47-58.
Medline Crossref
Pereira R, Sá R, Barros A, Sousa M. Major regulatory mechanisms involved in sperm motility. Asian J Androl. 2015. In Press.
Medline Crossref
Pinho
MJ, Neves R, Costa P, Ferrás C, Sousa M, Alves C, Almeida C, Fernandes
S, Silva J, Ferrás L, Barros A. Unique (Yq12;1q12) translocation with
loss of the heterochromatic region of chromosome 1 in a male with
azoospermia due to meiotic arrest. Hum Reprod. 2005; 20: 689-96.
Medline Crossref
Polyzos
NP, Blockeel C, Verpoest W, De Vos M, Stoop D, Vloeberghs V, Camus M,
Devroey P, Tournaye H. Live birth rates following natural cycle IVF in
women with poor ovarian response according to the Bologna criteria. Hum
Reprod. 2012; 27: 3481-6.
Medline Crossref
Practice
Committees of American Society for Reproductive Medicine, Society for
Assisted Reproductive Technology. Mature oocyte cryopreservation: a
guideline. Fertil Steril. 2013; 99:37-43.
Medline Crossref
Racowsky
C, Kovacs P, Martins WP. A critical appraisal of time-lapse imaging for
embryo selection: where are we and where do we need to go? J Assist
Reprod Genet. 2015; 32: 1025-30.
Medline Crossref
Rienzi
L, Cobo A, Paffoni A, Scarduelli C, Capalbo A, Vajta G, Remohí J, Ragni
G, Ubaldi FM. Consistent and predictable delivery rates after oocyte
vitrification: an observational longitudinal cohort multicentric study.
Hum Reprod. 2012:6:1606–12.
Medline Crossref
Rodrigo
L, Mateu E, Mercader A, Cobo AC, Peinado V, Milán M, Al-Asmar N,
Campos-Galindo I, García-Herrero S, Mir P, Simón C, Rubio C. New tools
for embryo selection: comprehensive chromosome screening by array
comparative genomic hybridization. Biomed Res Int. 2014; 2014:517125.
Medline Crossref
Roque M. Freeze-all policy: is it time for that? J Assist Reprod Genet. 2015;32:171-6.
Medline Crossref
Sá
R, Cunha M, Rocha E, Barros A, Sousa M. Sperm DNA fragmentation is
related to sperm morphological staining patterns. Reprod BioMed Online.
2015; 31: 506-15.
Medline Crossref
Setti
AS, Paes de Almeida Ferreira Braga D, Iaconelli A Jr, Aoki T, Borges E
Jr. Twelve years of MSOME and IMSI: a review. Reprod Biomed Online.
2013;27:338-52.
Medline Crossref
Sousa
M, Lima M, Oliveira C, Cunha M, Teixeira da Silva J, Silva J, Viana P,
Barros A. Ovarian hyperstimulation syndrome: a clinical report on 4894
consecutive ART treatment cycles. Reprod Biol Endocrinol. 2015;13:66.
Medline Crossref
Sousa
M, Tesarik J. Ultrastructural analysis of fertilization failure after
intracytoplasmic sperm injection. Hum Reprod. 1994;9:2374-80.
Medline
Steptoe PC, Edwards RG. Birth after the reimplantation of a human embryo. Lancet. 1978; 2: 366.
Medline Crossref
Steward
R, Lan L, Shah A, Yeh JS, Price TM, Goldfarb JM, Muasher SJ.Oocyte
number as a predictor for ovarian hyperstimulation syndrome and live
birth: an analysis of 256,381 in vitro fertilization cycles. Fertil
Steril. 2014;101:967-73.
Medline Crossref
Stimpfel
M, Vrtacnik-Bokal E, Pozlep B, Virant-Klun I. Comparison of GnRH
agonist, GnRH antagonist, and GnRH antagonist mild protocol of
controlled ovarian hyperstimulation in good prognosis patients. Int J
Endocrinol. 2015;2015:385049.
Medline Crossref
Sunkara
SK, Rittenberg V, Raine-Fenning N, Bhattacharya S, Zamora J,
Coomarasamy A. Association between the number of eggs and live birth in
IVF treatment: an analysis of 400 135 treatment cycles. Hum Reprod.
2011;26:1768-74.
Medline Crossref
Tesarik J, Sousa M, Testart J. Human oocyte activation after intracytoplasmic sperm injection. Hum Reprod. 1994;9:511-8.
Medline
Tesarik
J, Sousa M. More than 90% fertilization rates after intracytoplasmic
sperm injection and artificial induction of oocyte activation with
calcium ionophore. Fertil Steril. 1995; 63: 343-9.
Medline
Timeva
T, Milachich T, Antonova I, Arabaji T, Shterev A, Omar HA. Correlation
between number of retrieved oocytes and pregnancy rate after in vitro
fertilization/intracytoplasmic sperm infection. Sc World J 2006;
6:686-90.
Medline Crossref
Tournaye
H, Liu J, Nagy Z, Verheyen G, Van Steirteghem A, Devroey P. The use of
testicular sperm for intracytoplasmic sperm injection in patients with
necrozoospermia. Fertil Steril. 1996; 66:331-4.
Medline
Tournaye
H, Verheyen G, Nagy P, Ubaldi F, Goossens A, Silber S, Van Steirteghem
AC, Devroey P. Are there any predictive factors for successful
testicular sperm recovery in azoospermic patients? Hum Reprod.
1997;12:80-6.
Medline Crossref
Van
Blerkom J, Henry G. Oocyte dysmorphism and aneuploidy in meiotically
mature human oocytes after ovarian stimulation. Hum Reprod.
1992;7:379-90.
Medline
Van
Steirteghem AC, Liu J, Joris H, Nagy Z, Janssenswillen C, Tournaye H,
Derde MP, Van Assche E, Devroey P. Higher success rate by
intracytoplasmic sperm injection than by subzonal insemination Report
of a second series of 300 consecutive treatment cycles. Hum Reprod.
1993a; 8:1055-60.
Medline
Van
Steirteghem AC, Nagy Z, Joris H. High fertilization and implantation
rates after intracytoplasmic sperm injection. Hum Reprod. 1993b;
8:1061-6.
Medline
Vanderzwalmen P, Fallet C. IMSI: indications, results and reflexions. J. Gynecol. Obstet. Biol. Reprod. 2010; 39: 22–5.
Medline Crossref
Vanderzwalmen
P, Hiemer A, Rubner P, Bach M, Neyer A, Stecher A, Uher P, Zintz M,
Lejeune B, Vanderzwalmen S, Cassuto G, Zech NH. Blastocyst development
after sperm selection at high magnification is associated with size and
number of nuclear vacuoles. Reprod Biomed Online. 2008;17: 617-27.
Medline Crossref
Vidal
F, Fugger EF, Blanco J, Keyvanfar K, Català V, Norton M, Hazelrigg WB,
Black SH, Levinson G, Egozcue J, Schulman JD. Efficiency of MicroSort
flow cytometry for producing sperm populations enriched in X- or
Y-chromosome haplotypes: a blind trial assessed by double and triple
colour fluorescent in-situ hybridization. Hum Reprod. 1998;13:308-12.
Medline Crossref
Waldby C.’Banking time’: egg freezing and the negotiation of future fertility. Cult Health Sex. 2015; 17:470-82.
Medline Crossref
Wang
WH, Meng L, Hackett RJ, Keefe DL. Developmental ability of human
oocytes with or without birefringent spindles imaged by Polscope before
insemination. Hum Reprod. 2001;16:1464-8.
Medline Crossref