JBRA Assist. Reprod. 2016;20 (3):132-136
ORIGINAL ARTICLE
doi: 10.5935/1518-0557.20160030
Prevalence of blood borne viruses in IVF: an audit of a fertility Centre
1Assisted Conception Unit, Lister Hospital & Fertility Centre, Accra, Ghana
2IVF Consultancy Services, Leicester, UK
CONFLICT OF INTERESTS
No conflict of interest have been declared.
ABSTRACT
Objective:
The rate of infertility continues to be on the increase in the
developing world. Similarly, the rates of blood-borne viral infections
(BBVs) such as Human Immunodeficiency Virus (HIV), Hepatitis B virus
(HBV) and Hepatitis C virus (HCV) are also on this rise. In 2014, the
World Health Organization (WHO) quoted prevalences of 1.5% (HIV), 15%
(HBV) 1.3 – 8.4% (HCV) in the Ghanaian general population. It has been
reported that BBVs can adversely affect male fertility, specifically
sperm count and progressive motility. The aim of this study was to
evaluate the prevalence of BBVs in people with infertility attending an
IVF clinic and whether or not BBVs impacted on sperm parameters.
Methods:
A retrospective cohort study at a private fertility center in Accra,
Ghana. We had 229 recruited couples assayed for HBV, HCV and HIV. Sperm
parameters of the male partners were also assessed. The analysis
performed included student t-test and Fisher’s exact test.
Results:
We found prevalence rates of 1.7% (HIV), 7.9% (HBV) and 0.4% (HCV),
which is similar to what has already been reported in the Ghanaian
community. There was no significant difference between BBV positive and
negative subjects for sperm count (13.6 million/ml vs. 17.7 million/ml,
P = 0.0599), percentage of progressive motility (26% vs. 30%, P = 0.2129), percentage of normal forms (3% vs. 3%, P = 0.0617) and clinical pregnancy rates per embryo transfer (36.1% vs 34.9%, P = 0.5) between BBV positive and BBV negative subjects, respectively.
Conclusion:
There is a similar prevalence of BBVs in sub-fertile couples and the
general Ghanaian population. However, no detrimental effect has been
reported for sperm parameters on grounds of BBV infectivity of the male
partner.
Keywords: Hepatitis B, Hepatitis C, HIV, sperm quality, IVF
INTRODUCTION
Worldwide more than 70 million, or 10% (Boivin et al., 2007) of couples, suffer from infertility, with a high proportion living in developing countries (Ombelet et al., 2008). The inability to have children can have negative emotional, psychological and social impacts on the lives of these people (Ombelet et al., 2008; Rouchou, 2013).
In general, secondary infertility has been shown to dominate over
primary infertility in most developing countries, and this is chiefly a
result of improperly treated sexually transmitted infections (STIs),
resulting in pelvic inflammatory disease (PID) (Larsen et al., 2006; Elussein et al., 2008).
It is estimated that 40 million people worldwide are living with
HIV/AIDS and approximately two thirds of those live in sub-Saharan
Africa (WHO, 2015). The World Health Organization (WHO) quotes a prevalence of 1.1 – 2.0% in the Ghanaian general population (WHO, 2015). The national sero-prevalence of HIV was estimated to be 1.5% in 2013 by the national AIDS control program in Ghana (Ghana Health Service, 2008).
HIV-infected people were initially discouraged from starting a family (Savasi et al., 2013).
However, with the introduction of antiretroviral therapies, life
expectancies and the quality of life has dramatically improved and many
infected people are now thinking of starting a family, usually via
assisted reproductive technology (ART) (Savasi et al., 2013).
Several studies have documented reduced fecundity in HIV-infected individuals compared to uninfected people (Glynn et al., 2000, Brocklehurst & French, 1998).
On the other hand, marital instability and polygamy, secondary to
infertility, may in turn increase the spread of HIV-1 infection (Ombelet et al., 2008).
The prevalence of chronic Hepatitis B virus (HBV) infection varies
widely according to geographical area. Sub-Saharan Africa is endemic in
HBV with an estimated 5–25% being chronic carriers (Candotti et al., 2007). HBV prevalence in Ghana has been estimated to be around 15% (Ghana Health Service, 2008).
The prevalence of the Hepatitis C Virus (HCV) has been reported to be
>1% in southern African countries, 1.7 - 27.5% in central Africa and
1.4 - 7% in West and East Africa (Candotti et al., 2003). The estimated serum-prevalence of HCV is 1.3–8.4% among blood donors in Ghana (Ampofo et al., 2002).
In general, BBV infections have been shown to contribute to male
infertility either by direct toxic effects on cells in the male
reproductive tract, and/or indirectly by causing a local inflammatory
or immunological reaction (Zhou et al., 2011). HBV infection has been reported to increase chromosomal instability in sperm and impair overall sperm quality (Huang et al., 2003, Huang et al., 2002). Furthermore, HBV has been linked to decreased sperm motility (Lorusso et al., 2010).
However, other studies have reported no significant difference in sperm
quality between HBV -serum-positive and -negative men (Zhou et al., 2011).
Reduced implantation and pregnancy rates have also been shown following
IVF treatment for people with HBV compared to age-matched controls (Pirwany et al., 2004).
The handling of potentially BBV-infected body fluids, gametes or
embryos is a risk to healthcare professionals, such as physicians,
nurses and embryologists. In addition, uninfected couples being treated
at the same time may be at risk of nosocomial contamination (Lesourd et al., 2000). This is why strict adherence to the testing of all people seeking ART for HIV, HBV and HCV is mandatory (The Commission of the European Communities, 2006; Practice Committee of American Society for Reproductive & Practice Committee of Society for Assisted Reproductive, 2008).
The aim of the current study was to calculate the BBV prevalence in
people seeking ART at a private fertility clinic in Accra, Ghana. We
also investigated if BBVs have any effect on sperm parameters.
MATERIALS AND METHODS
Subjects
were recruited between March 2013 and July 2015. Participating subjects
signed consent forms to participate in the study, which was approved by
the hospital’s ethics and practice committee.
Two hundred and twenty nine (229) heterosexual couples were recruited
for this study, having complete viral screening results (HIV, HBV,
HCV). Recipients of donor gametes (either sperm or oocyte) were
included, provided the other gamete originated from a BBV-infected
partner. Patients receiving frozen embryo transfers were excluded.
For BBV analysis, 5ml blood was collected by venipucture into serum
separator tubes and centrifuged at 500g for 5 minutes to separate the
serum from cells. HBsAg, Anti-HIV 1 and 2 and HCV were determined from
the serum with rapid diagnostics kits (Tellmefast, Biocan Diagnostics
Inc, Canada). Quality control checks were performed daily before
running assays.
The IVF stimulation protocol was as follows; down-regulation was
achieved with 0.5 units of buserelin administered from Day 2 of the
menstrual cycle till HCG administration. An ultrasound scan was
performed between 14 – 21 days after starting buserelin injections to
assess ovarian status and endometrial thickness. When down regulation
was achieved, controlled ovarian stimulation (COS) was initiated
alongside the buserelin administration. For the COS, 225 - 400 IU of
recombinant FSH (Fostimon, IBSA, Switzerland) was administered daily
for 7 – 10 days. An ultrasound scan was performed to assess follicular
response between 5 - 7 days of COS and dosage adjusted accordingly when
required. HCG (10,000 IU) (Choriomon, IBSA, Switzerland) was
administered when the leading follicle was at least 18mm.
Ultrasound-guided follicle aspiration was performed using a 17G Cook
aspiration needle (Cook, Australia) 36 hours after the HCG injection.
Semen samples produced on the day of the IVF/ICSI procedures were
analyzed according the latest WHO laboratory manual for the examination
and processing of human semen (Cooper et al., 2010).
The semen was prepared by the density gradient technique of sperm
preparation. 1ml 40% gradient was gently over-layered onto 1ml 80%
gradient (Global, IVF Online, Denmark) and warmed in the incubator set
to 37°C for 30 minutes. 1ml of the semen was gently over-layered on the
40% gradient and centrifuged at 300g for 10 minutes. The supernatant
was gently aspirated and discarded. About 0.3ml of the remaining pellet
was aspirated and transferred into 3ml of AllGrad sperm washing
solution (Global, IVF Online, Denmark) and centrifuged at 300g for 5
minutes. The supernatant was gently aspirated and discarded. A sperm
count and motility assessment was then performed on the washed pellet
using a sterile technique. This pellet was kept for use in the IVF
procedure.
Semen samples from BBV infected males were also processed using the
density gradient centrifugation method with three gradient layers 90%,
70% and 40%, under a sterile technique. The removal of the supernatant
at each step prior to transfer of the pellet helped minimize any viral
transmission (Zamora et al., 2016).
Developing embryos from BBV-positive couples were cultured in separate
gassed incubators (5% CO2, 6% O2 and 89% nitrogen, BOC, UK) from
BBV-negative couples, to eliminate any risk of cross-contamination as
per best practice (Magli et al., 2008).
A pregnancy test was performed on the serum of the female partners two weeks after the embryos were transferred.
Results were expressed as mean ± SEM or mean (range). The data was
analysed using the Graph Pad Prism - version 5 (Graph Pad Software, San
Diego California). Student t-test and Fisher’s exact test were used to
assess significance. Statistical significance was set at P<0.05
RESULTS
Twenty-three (23) couples had repeat IVF treatments. One (1) female
partner and four (4) male partners tested positive for HBsAg in both
cycles. Two (2) male partners who tested negative at the first IVF
cycle tested positive for HBsAg in the second, despite the advice and
the availability of the hepatitis B vaccine at the Public Health Unit
of the hospital. The time interval between both cycles was twelve (12)
months for one male and 21 months for the other. Primary infertility
was dominant over secondary infertility in our study population (Table 1).
HBV prevalence was higher in the study population compared to their HIV
and HCV infected counterparts. More men were significantly infected
with the HBV than women (P=0.0027).
The study did not find any significant difference in semen quality,
i.e. sperm count, percentage progressive motility and percentage normal
forms of BBV positive and BBV negative males. However, there was a
trend for mean sperm count and percentage progressive motility to be
higher in the BBV-negative males, although this was not significantly
different from BBV-positive males (Table 2).
On account of the high prevalence of HBV, the effect of this virus on semen was assessed separately (Table 3). However, we found no significant difference in semen quality between HBV-infected males and those not infected.
There was no statistical difference in semen quality when HIV infected males were compared to their HIV uninfected males (Table 4). All 5 males and the 3 females infected by the HIV were on the highly active anti-retroviral therapy (HAART).
There was only 1 male infected with the HCV hence comparative analysis
of semen quality between HCV positive and HCV negative males could not
be performed due to small numbers.
We did not find any significant difference for clinical pregnancy rates between BBV-infected and uninfected couples (Figure 1). Interestingly, BBV-infected women had slightly higher pregnancy rates than those without BBV infection (36.1% vs 34.9%, P = 0.5000, 1-tailed).
DISCUSSION
BBV (HIV, HBV and HCV) prevalence in our study population was similar to that found in other studies (Duda et al., 2005).
Techniques, such as the density gradient sperm preparation, have been
shown to significantly reduce the risk of transmission of viral
infection from parent to offspring, especially if the male is infected (Zafer et al., 2016).
With this, one might consider that BBV prevalence might be higher in an
IVF population than in the general population, since infected men can
take advantage of such procedures to prevent the risk of transmitting
the virus to partners and offspring. However, it is possible that
BBV-infected couples are still unaware of the benefits of such ART
techniques, and as such, have not taken the opportunity it offers them
to procreate without the risk of viral transmission to their offspring.
It is also possible that they are aware of these benefits but are
unable to pay for such services.
It has been reported that male
partners of infertile heterosexual relationships may have extra-marital
affairs in their quest to achieve pregnancy (Ombelet et al., 2008).
As such, they are more likely not to use any physical barrier
contraception such as condoms and, as a result, are more prone to
contract sexually transmitted infections such as BBVs (Ombelet et al., 2008). This is evident in the current study, since more males were infected with BBV than their female counterparts (Table 2).
Two male patients tested positive for HBsAg on their second attempt at
IVF/ICSI, although they were negative during their first attempt. As
per standard protocol, all patients who test negative for the Hepatitis
B virus are encouraged to receive the vaccination, which was available
at the study site. It seems that these two men did not utilize this
option. The female partners of these two men tested negative for HBsAg
in both cycles, since they received the vaccine after the first testing.
The study did not find any difference between the sperm quality of BBV
infected males and their uninfected counterparts. This supports data
from Zhou et al. (2011) who also reported no difference in sperm quality between HBV positive and negative males.
There was no significant difference in pregnancy rates between BBV
positive couples and their negative counterparts (36.1% vs. 34.9%, P
= 0.5000). The slight difference in favor of BBV-positive couples could
be due to their slightly younger age as compared to their BBV-negative
counterparts although again there was no significant difference in
their ages (35.9 ± 1.0 vs. 38.2 ± 0.5, P = 0.0564 respectively, Table 1).
We hypothesize that due to the relative high cost of IVF procedures,
there is the tendency that those who have no children (primary
infertility) will have a greater burden to seek ART services than those
with secondary infertility (Table 1).

Table 1. Demographics of the general populationmigration pattern.
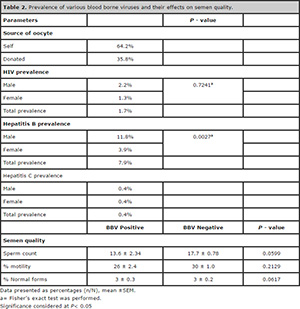
Table 2. Prevalence of various blood borne viruses and their effects on semen quality.
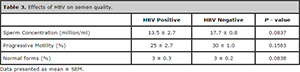
Table 3. Effects of HBV on semen quality.
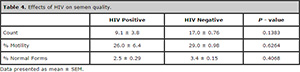
Table 4. Effects of HBV on semen quality.
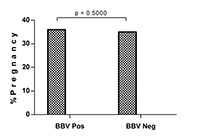
Figure 1. Pregnancy percentage differences by way of BBV infection.
CONCLUSION
Access to ARTs is gradually increasing, with about 15 fertility centers
in Ghana, although all centers are in the private sector. This current
study shows similar BBV prevalence rates in an IVF population and the
general population. This underscores the importance of a strict
adherence to pre-treatment testing for such viruses, to ensure the
safety of personnel and gametes of uninfected patients in these
fertility centers. On the basis of viral infectivity, there was no
known effect on semen quality. It is, however, noteworthy that these
participants were on HAART and these medications could have minimized
any adverse effect on semen quality in HIV infected males. A broader
study is required to assess semen quality of HIV infected males with
and without HAART.
REFERENCES
Ampofo
W, Nii-Trebi N, Ansah J, Abe K, Naito H, Aidoo S, Nuvor V, Brandful J,
Yamamoto N, Ofori-Adjei D, Ishikawa K. Prevalence of blood-borne
infectious diseases in blood donors in Ghana. J Clin Microbiol.
2002;40:3523-5.
Medline Crossref
Boivin
J, Bunting L, Collins JA, Nygren KG. International estimates of
infertility prevalence and treatment-seeking: potential need and demand
for infertility medical care. Hum Reprod. 2007;22:1506-12.
Medline Crossref
Brocklehurst
P, French R. The association between maternal HIV infection and
perinatal outcome: a systematic review of the literature and
meta-analysis. Br J Obstet Gynaecol. 1998; 105:836-48.
Medline Crossref
Candotti
D, Danso K, Allain JP. Maternofetal transmission of hepatitis B virus
genotype E in Ghana, west Africa. J Gen Virol. 2007;88:2686-95.
Medline Crossref
Candotti
D, Temple J, Sarkodie F, Allain JP. Frequent Recovery and Broad
Genotype 2 Diversity Characterize Hepatitis C Virus Infection in Ghana,
West Africa. J Virol. 2003; 77:7914-23.
Medline Crossref
The Commission of the European Communities. Commission directive 2006/17/EC. Implementing Directive 2004/23/EC of the European Parliament and of the Council as regards certain technical requirements for the donation, procurement and testing of human tissues and cells. Official Journal of the European Union. Link: http://eur-lex.europa.eu/eli/dir/2006/17/oj
Cooper
TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM, Haugen
TB, Kruger T, Wang C, Mbizvo MT, Vogelsong KM. World Health
Organization reference values for human semen characteristics. Hum
Reprod Update. 2010;16:231-45.
Medline Crossref
Duda
RB, Darko R, Adanu RM, Seffah J, Anarfi JK, Gautam S, Hill AG. HIV
prevalence and risk factors in women of Accra, Gghana: results from the
women’s health study of Accra. Am J Trop Med Hyg. 2005; 73:63-6.
Medline
Elussein
EA, Magid YM, Omer MM, Adam I. Clinical patterns and major causes of
infertility among Sudanese couples. Trop Doct. 2008;38:243-4.
Medline Crossref
Ghana Health Service. Guidelines for Antiretroviral therapy in Ghana. 2008. Available at: http://www.who.int/hiv/pub/guidelines/ghana_art.pdf?ua=1
Glynn
JR, Buvé A, Caraël M, Kahindo M, Macauley IB, Musonda RM, Jungmann E,
Tembo F, Zekeng L. Decreased fertility among HIV-1-infected women
attending antenatal clinics in three African cities. J Acquir Immune
Defic Syndr. 2000;25:345-52.
Medline
Huang
JM, Huang TH, Qiu HY, Fang XW, Zhuang TG, Liu HX, Wang YH, Deng LZ, Qiu
JW. Effects of hepatitis B virus infection on human sperm chromosomes.
World J Gastroenterol. 2003;9:736-40.
Medline Crossref
Huang
JM, Huang TH, Qiu HY, Fang XW, Zhuang TG, Qiu JW. Studies on the
integration of hepatitis B virus DNA sequence in human sperm
chromosomes. Asian J Androl. 2002; 4:209-12.
Medline
Larsen
U, Masenga G, Mlay J. Infertility in a community and clinic-based
sample of couples in Moshi, Northern Tanzania. East Afr Med J.
2006;83:10-7.
Medline Crossref
Lesourd
F, Izopet J, Mervan C, Payen JL, Sandres K, Monrozies X, Parinaud J.
Transmissions of hepatitis C virus during the ancillary procedures for
assisted conception. Hum Reprod. 2000;15:1083-5.
Medline Crossref
Lorusso
F, Palmisano M, Chironna M, Vacca M, Masciandaro P, Bassi E, Selvaggi
Luigi L, Depalo R. Impact of chronic viral diseases on semen
parameters. Andrologia. 2010;42:121-6.
Medline Crossref
Magli
MC, Van den Abbeel E, Lundin K, Royere D, Van der Elst J, Gianaroli L;
Committee of the Special Interest Group on Embryology. Revised
guidelines for good practice in IVF laboratories. Hum Reprod. 2008;
23:1253-62.
Medline Crossref
Ombelet
W, Cooke I, Dyer S, Serour G, Devroey P. Infertility and the provision
of infertility medical services in developing countries. Hum Reprod
Update. 2008;14:605-21.
Medline Crossref
Pirwany
IR, Phillips S, Kelly S, Buckett W, Tan SL. Reproductive performance of
couples discordant for hepatitis B and C following IVF treatment. J
Assist Reprod Genet. 2004; 21:157-61
Medline Crossref
Practice
Committee of American Society for Reproductive Medicine; Practice
Committee of Society for Assisted Reproductive Technology. Revised
guidelines for human embryology and andrology laboratories. Fertil
Steril. 2008; 90: S45-59.
Medline Crossref
Rouchou B. Consequences of infertility in developing countries. Perspect Public Health. 2013; 133:174-9
Medline Crossref
Savasi V, Mandia L, Laoreti A, Cetin I. Reproductive assistance in HIV serodiscordant couples. Hum Reprod Update. 2013;19:136-50
Medline Crossref
WHO. Country factsheet. 2015. Available at: http://aidsinfo.unaids.org/. Accessed: 25/08/2015.
Zafer
M, Horvath H, Mmeje O, van der Poel S, Semprini AE, Rutherford G, Brown
J. Effectiveness of semen washing to prevent human immunodeficiency
virus (HIV) transmission and assist pregnancy in HIV-discordant
couples: a systematic review and meta-analysis. Fertil Steril.
2016;105:645-55.
Medline Crossref
Zamora
MJ, Obradors A, Woodward B, Vernaeve V, Vassena R. Semen residual viral
load and reproductive outcomes in HIV-infected men undergoing ICSI
after extended semen preparation. Reprod Biomed Online. 2016;32:584-90.
Medline Crossref
Zhou
XP, Hu XL, Zhu YM, Qu F, Sun SJ, Qian YL. Comparison of semen quality
and outcome of assisted reproductive techniques in Chinese men with and
without hepatitis B. Asian J Androl. 2011;13:465-9.
Medline Crossref