JBRA Assist. Reprod. 2024;28(2):341-348
REVIEW
doi: 10.5935/1518-0557.20240013
Probiotics supplementation in the treatment of male infertility: A Systematic Review
1Department of Pharmaceutical Sciences. University of Vila Velha - UVV. Vila Velha, Espírito Santo, Brazil
CONFLICT OF INTEREST
The authors have nothing to declare.
ABSTRACT
Infertility is a widespread global issue that affects approximately 15% of sexually active and active couples, which contributes to about 50% of cases. Currently, the condition remains prevalent and often inadequately treated. This systematic review aims to evaluate existing studies investigating the effects of probiotic supplementation in men. A comprehensive search was conducted across major databases, including PubMed, Cochrane, Science Direct, and Scielo, using relevant keywords such as ‘probiotic’ OR ‘Lactobacillus’ OR ‘Bifidobacterium’ AND ‘Male infertility’ OR ‘male fertility’ OR ‘sperm quality’ OR ‘sperm motility’ OR ‘oligoasthenoteratozoospermia’ and their Portuguese equivalents. Four randomized clinical studies met the inclusion criteria, focusing on men diagnosed with idiopathic male infertility (oligozoospermia, teratozoospermia, and asthenozoospermia). The findings revealed that probiotic administration exhibited promising antioxidant properties by combating reactive oxygen species (ROS), consequently protecting sperm DNA from damage that correlates with declining sperm quality. Significant improvements were observed across all sperm parameters, with notable enhancement in motility. Consequently, probiotic supplementation emerges as a potential therapeutic alternative for men diagnosed with idiopathic infertility, demonstrating positive effects on sperm quality.
Keywords: oligoasthenoteratozoospermia, DNA fragmentation, oxidative stress, Lactobacillus, Bifidobacterium
INTRODUCTION
Infertility is defined as the inability of a couple to conceive spontaneously after one year of having sexual intercourse without using any contraceptive method. Corresponding to the period that most couples get pregnant spontaneously, this does not imply, however, that the investigation of infertility is postponed until the 12-month period has elapsed (Rowe et al., 1993).
This problem affects approximately 15% of sexually active couples and about 7% of men worldwide (Nieschlag et al., 2010). The causes of infertility are several, caused by problems in the female or male reproductive physiology. However, the male factor contributes in 50% of cases, standing out as the only cause in approximately 20 to 30% of cases (Agarwal et al., 2015).
In the male reproductive system, infertility is any dysfunction in the ejection and quality of semen, whether characterized by the absence, reduced quantity, or changes in sperm morphology or motility (Danis & Samplaski, 2019; Gatimel et al., 2017). According to Krzastek et al. (2020), approximately 44% of infertile men are diagnosed as idiopathic, i.e., they have no definite cause.
Moreover, Ray et al. (2017) point out that in the majority of male infertility cases, the abnormality is only observed after semen analysis. From sperm analysis and observation of spermatozoa, it is possible to determine whether there is a change in the number (oligozoospermia), motility (asthenozoospermia), or the presence of abnormal forms (teratozoospermia) of gametes. These abnormalities, usually present together, are called oligoasthenoteratozoospermia syndrome (OAT).
Currently, it is known that male infertility has a multifactorial origin and may result from factors such as the use of drugs and medication, endocrine and hormonal disorders, environmental pollutants, smoking, urogenital tract infections, alcohol abuse, smoking, exposure to chemical substances, among others (Brincat et al., 2015; Dissanayake et al., 2019; Mann et al., 2020). Despite the multifactorial feature in male infertility, significant evidence points to its strong correlation with seminal oxidative stress (Costa et al., 2023). Oxidative stress is a condition of seminal physiological imbalance due to an increase in reactive oxygen species (ROS) or a deficiency in total antioxidant capacity (TAC) (Pizzino et al., 2017).
Elevated levels of ROS can lead to a sequence of events that lead to cellular damage to sperm lipids, DNA, and proteins (Ayaz et al., 2018), which reduces its ability to move and fertilize. Studies show that infertile men may have elevated levels of ROS and less effective semen antioxidant capacity (Lewis et al., 1997; Subramanian et al., 2018).
Wang et al. (2022) point out that, despite efforts to identify the causes and develop new approaches to improve male fertility in recent decades, there are still few effective treatments to delay the decline of idiopathic male infertility. Thus, recent studies have evaluated different antioxidant supplementation strategies and their effects on ROS to improve male infertility (Bozhedomov et al., 2017; Kızılay & Altay, 2019; Kopets et al., 2020) and it has been shown that natural antioxidants or vitamin supplements, like vitamins C and E, glutathione, folate, zinc, selenium, carnitine, coenzyme Q10, lycopene, and N-acetyl cysteine, can neutralize free radicals thus improving semen parameters (Bui et al., 2018; Busetto et al., 2018; Gamidov et al., 2019).
However, other approaches that do not use antioxidants but can also promote antioxidant action have been gaining prominence. In this sense, the use of probiotics has grown, chiefly due to their antioxidant action and improvement in the seminal microbiota (Helli et al., 2022). Probiotics are defined as “live microorganisms which, when administered in adequate amounts, confer a health benefit on the host” (FAO/WHO, 2001). The most common strains belong to the genera Lactobacillus and Bifidobacterium, which are lactic acid producers and are part of many industrial and artisanal fermentations of plants, meat, and dairy products (Bolivar-Jacobo et al., 2023; Damián et al., 2022).
Microbiota describes the community of microbes that reside in almost every part of the human body. The microbiome refers to the genetic material of the microbiota, which plays roles in physiological symbiosis and pathogenic dysbiosis (Lundy et al., 2021). Much of the human microbiota is housed in the gastrointestinal tract, often considered an additional organ (Venneri et al., 2022). The dominant bacteria of the intestinal microbiota are represented by the phyla Firmicutes and Bacteroidetes, followed by the phyla Actinobacteria, and Proteobacteria, which are part of the composition of a healthy intestinal microbiota and contribute to the maintenance and protection of the intestinal epithelium (Eckburg et al., 2005; Rinninella et al., 2019).
Already in the seminal microbiome, the dominant phyla are Proteobacteria, Firmicutes, Actinobacteria, and Bacteroidetes (Yang et al., 2020). Lundy et al. (2021) found that Prevotella abundance was negatively associated with sperm concentration, as opposed to Pseudomonas, which was positively associated with motile sperm count. Additionally, increased abundance of Ureaplasma, Enterococcus, Mycoplasma, and Prevotella were negatively associated with sperm parameters. In addition, evidence shows that Lactobacillus can promote a protective action on semen quality, with a higher presence of this microorganism observed in samples of normal morphology (Farahani et al., 2021).
Thus, due to the potential of probiotics to promote the balance of the microbiome, as well as their antioxidant capacity, which has a strong association with male fertility (Wang et al., 2022), the objective of this systematic review is to evaluate available studies that have investigated the effects of probiotic supplementation on male infertility.
MATERIAL AND METHODS
A systematic search of articles was conducted between February and March 2022, encompassing both English and Portuguese and without any restriction on publication date. The databases used for the search included PubMed, Cochrane (Central Register of Controlled Trials), Science Direct, and Scielo. The search terms employed were ‘Probiotic’, ‘Lactobacillus’, ‘Bifidobacterium’, ‘Male fertility’, ‘Male infertility’, ‘sperm quality’, ‘sperm motility’, and ‘Oligoasthenoteratozoospermia’. The English equivalents of these terms were combined as follows: ((probiotic) OR (Lactobacillus) OR (Bifidobacterium)) AND ((“Male infertility”) OR (“male fertility”) OR (“sperm quality”) OR (“sperm motility”) OR (oligoasthenoteratozoospermia))), with adjustments made accordingly for each database.
The articles identified underwent an initial assessment based on their titles and abstracts. Those that satisfied the selection criteria were then read in their entirety. The eligibility criteria included original published studies involving human subjects and randomized clinical trials. Studies conducted on animal models or in vitro, review articles, and duplicate studies were excluded.
The key information from the articles was entered into a spreadsheet created using the Microsoft Excel® program. The extracted details included the author’s name, publication year, study design, study population, number of participants, duration of follow-up, intervention employed, and the primary findings obtained.
RESULTS AND DISCUSSION
Our review aimed to evaluate the effects of probiotic supplementation on male infertility. The systematic search in the selected databases resulted in 85 articles. After reading the titles, abstracts, and exclusions by eligibility criteria, four articles were included in this systematic review (Figure 1). Studies have shown that the use of probiotics for male infertility treatment promotes improvement in sperm parameters, such as motility, sperm concentration, morphology, semen volume, and total sperm count, thus being a relevant strategy in the therapy of male infertility.
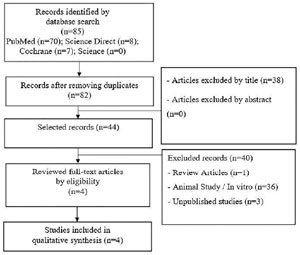
Figure 1. Flowchart the selection of studies for inclusion in the review.
Details of the articles included are in Table 1. The studies were published between 2017 and 2021. All were randomized clinical trials, two of which were double-blind (Helli et al., 2022; Maretti & Cavallini, 2017), a triple-blind (Abbasi et al., 2021), and a crossover study (Valcarce et al., 2017). All studies were performed in men with idiopathic male infertility (oligozoospermia, teratozoospermia, and asthenozoospermia). The potential effects of probiotics have been studied in several areas of medicine. Mixtures of probiotic strains have shown to be beneficial in several outcomes, such as irritable bowel syndrome, diarrhea, atopy, respiratory tract infections, modulation of the immune system and intestinal microbiota, and inflammatory bowel disease, among others (Chapman et al., 2011; La Fata et al., 2018). Considering its safe use and potential therapeutic benefits, its antioxidant properties have also been gaining attention in research lines) (Mishra et al., 2015; De Marco et al., 2018).
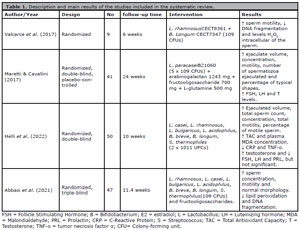
Table 1. Description and main results of the studies included in the systematic review.
The action mechanisms of probiotics may vary between strains and the result of a combination of events. In general, it is proposed that the main mechanisms consist of the production of antimicrobial enzymes or metabolites, inhibition of pathogenic bacteria due to competition for binding sites, competition for nutrients and modulation of the immune response (Saad et al., 2013; Fan & Pedersen, 2021).
In a review study, Wang et al. (2017) showed that probiotic strains can exert antioxidant action in different ways, including their ability to chelate metal ions, because they have their own antioxidases enzymes, they produce antioxidant metabolites, positively regulate the host’s antioxidant enzymatic activities and negatively regulate the activities of ROS production enzymes, increase the levels of metabolites with antioxidant action in their host, regulate signaling pathways and regulate the intestinal microbiota.
In the field of female reproduction, studies have shown that the use of probiotics influences the vaginal microbiome. The benefits of probiotics as a therapy for bacterial vaginosis, increasing the abundance of Lactobacilli, and being compelling in preparing the vaginal microbiome before conception have been reported (Macklaim et al., 2015; Abbe & Mitchell, 2023; Mashatan et al., 2023). Regarding male infertility, studies have shown that using probiotics improves sperm parameters and is indicated as a therapy for these patients (Maretti & Cavallini, 2017; Valcarce et al., 2017; Helli et al., 2022; Abbasi et al., 2021).
Among the investigated parameters, we also have the lowest sperm DNA fragmentation (Valcarce et al., 2017; Abbasi et al., 2021), as well as the reduction of ROS levels (Valcarce et al., 2017), reduced sperm lipid peroxidation (Abbasi et al., 2021), reduction in malondialdehyde (MDA) levels and increase in TAC (Helli et al., 2022).
Sperm parameters
All four articles included in the present review reported improvement in sperm motility (Maretti & Cavallini, 2017; Valcarce et al., 2017; Helli et al., 2022; Abbasi et al., 2021). Valcarce et al. (2017) demonstrated a significant improvement in motility after administering a probiotic (Lactobacillus rhamnosus CECT8361 + Bifidobacterium longum CECT7347) in 9 asthenozoospermic men for six weeks. The percentage of motile sperm increased about six-fold after treatment and was sustained for six weeks after the completion of treatment.
In agreement with these results, Maretti & Cavallini (2017) reported a significant increase in progressive sperm motility (p<0.01) in a randomized study of 41 men with idiopathic oligoasthenoteratospermia. Supplementation was performed with probiotic Flortec®, which contained Lactobacillus paracasei B21060 (5 x 109 CFUs) associated with the vehicle (arabinogalactan 1243 mg + fructooligosaccharide 700 mg + L-glutamine 500 mg). In addition to the improvement in motility, other parameters were also improved, such as sperm concentration (p<0.01), morphology (p<0.01), semen volume (p<0.01), and total sperm count (p<0.01).
Following the same trend, the results found by Helli et al. (2022), demonstrated that daily supplementation of strains Lactobacillus casei, Lactobacillus rhamnosus, Lactobacillus bulgaricus, Lactobacillus acidophilus, Bifidobacterium breve, Bifidobacterium longum, Streptococcus thermophiles, (2 x 1011 CFUs), for ten weeks, significantly improved ejaculate volume (p=0.041), total sperm count (p=0.001), concentration (p=0.001), total motility (p=0.037) and the number of live sperm (p=0.003).
In the same way, Abbasi et al. (2021) noted a significant increase in motility (p=0.03) and improvement in sperm concentration (p=0.004) and morphology (p=0.014) parameters after supplementation with FamiLact® (Lactobacillus rhamnosus, Lactobacillus casei, Lactobacillus, bulgaricus, Lactobacillus acidophilus, Bifidobacterium breve, Bifidobacterium longum, Streptococcus thermophilus (109 CFU) and fructooligosaccharides) for 80 days in 47 individuals. These modifications were attributed to improvement in oxidative stress and DNA damage.
DNA Fragmentation Index and oxidative stress
Valcarce et al. (2017) demonstrated a significant reduction (p<0.05) in the DNA Fragmentation Index (DFI) from 25.74% to 21.11% after three weeks (T1) and 21.58% after six weeks of treatment (T2), being correlated with improvement in motility. After the washout, the improvement in DFI was not sustained, increasing to 21.64% three weeks after the end of treatment (W1) and 23.09% after six weeks (W2).
Abbasi et al. (2021) observed a favorable improvement in DNA fragmentation (p=0.005) in patients who received FamiLact®. In the placebo group, a decrease in post-treatment DNA fragmentation was also observed, however, to a lesser extent (p=0.03). Concomitantly, the staining technique with A3 chromatin (CMA3) was performed, with no significant change in CMA3 positivity (p=0.03).
During spermatogenesis, chromatin undergoes remodeling, replacing nuclear histones with protamine, a positively charged molecule. This change enhances DNA stability and reduces damage susceptibility (Bennetts & Aitken, 2005). Abbasi et al. (2021) propose that the elevated protamine levels might not be the sole reason for reduced DNA fragmentation. Instead, their study supports the hypothesis that probiotics’ ability to lower ROS levels contributes to the prevention of DNA damage, thus highlighting their potential as antioxidants.
Oxidative stress was assessed by three of the four studies included in this research (Valcarce et al., 2017; Helli et al., 2022; Abbasi et al., 2021), demonstrating that probiotics promote antioxidant action. A study performed by Valcarce et al. (2017), using an in vitro model to analyze the antioxidant action of probiotics in Caenorhabditis elegans, demonstrated a higher percentage of worm survival after incubation with the strains (L. rhamnosus CECT8361 58.5% and B. longum OCECT7347 66%) of that of the positive control replicas, using vitamin C, thus confirming the antioxidant activity of the two strains.
Intracellular H2O2 levels were also analyzed to confirm that the antioxidant properties of probiotics in preventing DNA fragmentation would be a consequence of the reduction of ROS. The dichlorodihydrofluorescein assay (DCFH-DA) was used to measure H2O2 levels, with the percentage of positive cells reduced in relation to the control (16.57±3.34%) after probiotic ingestion in T1 (5.02±0.93%) and T2 (6.2±1.77%), remaining even after washout W1 (7.27±2.37%) and W2 (7.9±1.36%) (Valcarce et al., 2017).
Helli et al. (2022) evaluated serum and seminal MDA levels, a lipid oxidation product, and used a colorimetric method to analyze serum and seminal CAT. As a result, they found that TAC and MDA levels in plasma and semen in the intervention group significantly changed compared to placebo (p<0.001 and p=0.002, respectively). Additionally, Abbasi et al. (2021) used a BODIPY probe to evaluate sperm lipid peroxidation and found a significant reduction (29.53%±19.4 to 26%±18.82; p=0.02) after supplementation.
A previous in vitro study by Barbonetti et al. (2011) provided initial evidence supporting the effectiveness of probiotics in safeguarding spermatozoa against lipid peroxidation and its detrimental impact on sperm motility and viability. The study employed a combination of three Lactobacillus strains. In a separate study conducted on rats fed a high-fat diet, significant reductions were observed in the activities of superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) in both serum and sperm, compared to control rats. This group exhibited elevated levels of malondialdehyde (MDA) and nitric oxide (NO), indicating the presence of oxidative stress and lipid peroxidation. However, supplementation with probiotics restored SOD and GSH-Px activities and reduced MDA and NO levels, approaching those of the control group. That demonstrates the antioxidant properties of probiotics and their ability to mitigate oxidative damage to some extent. It has been established that excessive free radicals can diminish sperm count, viability, and motility (Chen et al., 2013).
It is known that defective spermatozoa are more vulnerable to damage caused by oxidative stress, because the composition of their plasmatic membranes contains high percentages of polyunsaturated fatty acids (PUFAs). This condition favors the generation of ROS by sperm mitochondria, inducing an increase in lipid peroxidation production process by the sperm in addition to the lack of cytoplasmic enzyme system. In addition to lacking cytoplasmic enzyme systems necessary for repairing damage induced by oxidative stress (Agarwal et al., 2003; Aitken et al., 2010; Darbandi et al., 2018).
ROS (Reactive Oxygen Species) inflict initial damage to the sperm membrane, consequently impairing its motility and ability to fuse with the oocyte (Darbandi et al., 2018). The precise mechanisms underlying reduced sperm motility due to oxidative stress remain uncertain. However, studies suggest that it may be attributed to axonemic alterations resulting from potential depletion of intracellular adenosine triphosphate (ATP), as well as tail abnormalities leading to decreased sperm motility (Ayaz et al., 2018; de Lamirande & Gagnon, 1992).
Oxidative stress can also cause DNA damage, accelerating the cell apoptosis process and consequent decrease in sperm count, being correlated with infertility and decreased semen quality (Agarwal et al., 2003; Ayaz et al., 2018; Sumner et al., 2019).
Such evidence and results found in our review reinforce this hypothesis that the administration of probiotics exerts an antioxidant action, acting in defense against ROS and damage to sperm DNA, with consequent improvement in sperm parameters, with emphasis on motility.
Sex hormones
Two studies (Maretti & Cavallini, 2017; Helli et al., 2022) evaluated blood levels of sex hormones after intervention with probiotics. Maretti & Cavallini (2017) observed that follicle-stimulating hormone (FSH), luteinizing hormone (LH), and testosterone (T) levels increased (p<0.01), estradiol (E2) and prolactin (PRL) levels did not increase significant changes. On the other hand, Helli et al. (2022) observed increased testosterone levels and decreased serum levels of FSH, LH, and PRL. However, these findings were not statistically significant (p=0.063, p=0.21 p=0.109, and p=0.128, respectively). These results, although inconclusive, suggest that the use of probiotics may influence hormonal regulation.
Hurtado de Catalfo et al. (2007) suggested that supplementation with antioxidants can improve the clinical picture of men with varicocele. Authors suggest that hormonal alterations may be associated with an indirect effect of ROS on the action of Leydig cells, mainly on the regulation of testosterone levels (Darbandi et al., 2018). Furthermore, evidence had already shown that oxidative stress is capable of inhibiting steroidogenesis in these cells (Mancini et al., 2023).
Thus, the hypothesis is that increased levels of testosterone and changes in gonadotropins may be related to the reduction of oxidative stress. Since the results are controversial and inconclusive, the physiological significance of the alterations needs to be clarified and the real impact of probiotics on hormonal factors evaluated.
Inflammatory factors
Regarding inflammatory factors, these were investigated only by Helli et al. (2022), who determined serum levels of tumor necrosis factor α (TNFα) and C-Reactive Protein (CRP), demonstrating that after probiotic supplementation, there was a significant reduction in both CRP and TNFα (p=0.001 and p=0.003, respectively). Similarly, Chen et al. (2012) investigated the in vivo effects of the Lactobacillus kefiranofaciens M1 strain on the intestinal epithelial cells of mice with colitis induced by Dextran sodium sulfate (DSS), and as a result, they observed that the use of the strain significantly reduced the production of pro-inflammatory cytokines (IL-1β and TNF-α) and increased production of the anti-inflammatory cytokine (IL-10), thus suggesting the potential anti-inflammatory activity of the strain.
Nanda Kumar et al. (2008) in a previous study of mice treated with DSS, suggested that probiotic strains can change the profile of cytokine secretion from pro-inflammatory to anti-inflammatory. Thus, the evidence and results found in our review demonstrate the potential anti-inflammatory action of probiotics.
In addition, considerable advances in studies of the microbiota associated with the intestinal and genital tracts have been correlating microbial communities and the effects of bacterial dysbiosis on infertility (Venneri et al., 2022). Studies reviewed by Farahani et al. (2021) reported a greater abundance of Prevotella and Staphylococcus in semen, negatively correlating with motility. Abnormal sperm morphology was correlated with decreased Lactobacillus. Likewise, Lundy et al. (2021) demonstrated that the testicular microbiome may play a significant role in spermatogenesis and that intestinal and urinary dysbiosis may be a factor that influences infertility.
Monteiro et al. (2018) found an increased amount of Pseudomonas, Klebsiella, Aerococcus, Actinobaculum, Neisseria and a lower presence of Lactobacillus in groups with oligoastenoteratozoospermia and hyperviscosity. A lower prevalence of Lactobacillus and Propionibacterium was also observed. These are Gram-positive bacteria, which seem to affect maintaining semen quality, as well as the potential to lessen the negative influence of gram-negative bacteria like Prevotella, Aggregatibacter, and Pseudomonas (Weng et al., 2014).
The intestinal epithelial cell layer acts as the primary barrier for molecule permeation, allowing the passage of paracellular or transcellular molecules. Modifications in membrane composition affect this barrier, leading to changes in intestinal permeability (Ghosh et al., 2021). Besides safeguarding the host against infections and pathogens in the gastrointestinal tract, the intestinal barrier facilitates the proper absorption of nutrients from food and fluids (Slifer & Blikslager, 2020).
Probiotics have been found to impact intestinal pathophysiology in various ways, with compelling evidence suggesting their ability to enhance intestinal barrier function. That is achieved through the activation of genes involved in the formation of healthy tight junctions, which serve as adhesive connections between intestinal epithelial cells (La Fata et al., 2018). In vitro and in vivo studies conducted by Blackwood et al. (2017) demonstrated that specific strains of Lactobacillus probiotics strengthen the intestinal barrier and maintain tight junction integrity.
However, it is relevant to note that the study mentioned has certain limitations, including a limited number of available articles on the subject and small sample sizes. Despite variations in probiotic administration, composition, dosage, and duration among studies, all have shown promising results in improving sperm parameters and potential treatment of male infertility.
CONCLUSION
The findings in our review demonstrate the benefit of probiotics treatment in male infertility. Confirming the results of improvement in sperm parameters, such as motility, sperm concentration, morphology, semen volume, and total sperm count, after treatment with probiotic strains (Lactobacillus, Bifidobacterium, and Streptococcus). It is considered a safe and affordable treatment. The still limited number of studies available on the use of probiotic therapy as an intervention in male infertility is highlighted, and it is suggested that larger-scale studies be performed to elucidate the mechanism of action and application of probiotics.
REFERENCES
Abbasi B, Abbasi H, Niroumand H. Synbiotic (FamiLact) administration in idiopathic male infertility enhances sperm quality, DNA integrity, and chromatin status: A triple-blinded randomized clinical trial. Int J Reprod Biomed. 2021;19:235-44. PMID: 33842820 DOI: 10.18502/ijrm.v19i3.8571 Medline
Abbe C, Mitchell CM. Bacterial vaginosis: a review of approaches to treatment and prevention. Front Reprod Health. 2023;5:1100029. PMID: 37325243 DOI: 10.3389/frph.2023.1100029 Medline
Agarwal A, Mulgund A, Hamada A, Chyatte MR. A unique view on male infertility around the globe. Reprod Biol Endocrinol. 2015;13:37. PMID: 25928197 DOI: 10.1186/s12958-015-0032-1 Medline
Agarwal A, Saleh RA, Bedaiwy MA. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril. 2003;79:829-43. PMID: 12749418 DOI: 10.1016/S0015-0282(02)04948-8 Medline
Aitken RJ, Baker MA, De Iuliis GN, Nixon B. New insights into sperm physiology and pathology. Handb Exp Pharmacol. 2010;(198):99-115. PMID: 20839089 DOI: 10.1007/978-3-642-02062-9_7 Medline
Ayaz A, Agarwal A, Sharma R, Kothandaraman N, Cakar Z, Sikka S. Proteomic analysis of sperm proteins in infertile men with high levels of reactive oxygen species. Andrologia. 2018;50:e13015. PMID: 29656391 DOI: 10.1111/and.13015 Medline
Barbonetti A, Cinque B, Vassallo MR, Mineo S, Francavilla S, Cifone MG, Francavilla F. Effect of vaginal probiotic lactobacilli on in vitro-induced sperm lipid peroxidation and its impact on sperm motility and viability. Fertil Steril. 2011;95:2485-8. PMID: 21497805 DOI: 10.1016/j.fertnstert.2011.03.066 Medline
Bennetts LE, Aitken RJ. A comparative study of oxidative DNA damage in mammalian spermatozoa. Mol Reprod Dev. 2005;71:77-87. DOI: 10.1002/mrd.20285 PMID: 15736137 DOI: 10.1002/mrd.20285 Medline
Blackwood BP, Yuan CY, Wood DR, Nicolas JD, Grothaus JS, Hunter CJ. Probiotic Lactobacillus Species Strengthen Intestinal Barrier Function and Tight Junction Integrity in Experimental Necrotizing Enterocolitis. J Probiotics Health. 2017;5:159. PMID: 28638850 DOI: 10.4172/2329-8901.1000159 Medline
Bozhedomov VA, Lipatova NA, Bozhedomova GE, Rokhlikov IM, Shcherbakova EV, Komarina RA. [Using L- and acetyl-L-carnintines in combination with clomiphene citrate and antioxidant complex for treating idiopathic male infertility: a prospective randomized trial]. Urologiia. 2017;(3):22-32. Russian. DOI: 10.18565/urol.2017.3.22-32 PMID: 28845935 DOI: 10.18565/urol.2017.3.22-32 Medline
Brincat D, Catania S, Wismayer PS, Calleja-Agius J. Male factors in ART outcome prediction. Gynecol Endocrinol. 2015;31:169-75. DOI: 10.3109/09513590.2014.984678 PMID: 25430662 DOI: 10.3109/09513590.2014.984678 Medline
Bui AD, Sharma R, Henkel R, Agarwal A. Reactive oxygen species impact on sperm DNA and its role in male infertility. Andrologia. 2018;50:e13012. PMID: 29644708 DOI: 10.1111/and.13012 Medline
Busetto GM, Agarwal A, Virmani A, Antonini G, Ragonesi G, Del Giudice F, Micic S, Gentile V, De Berardinis E. Effect of metabolic and antioxidant supplementation on sperm parameters in oligo-astheno-teratozoospermia, with and without varicocele: A double-blind placebo-controlled study. Andrologia. 2018;50. PMID: 29315686 DOI: 10.1111/and.12927 Medline
Chapman CM, Gibson GR, Rowland I. Health benefits of probiotics: are mixtures more effective than single strains? Eur J Nutr. 2011;50:1-17. PMID: 21229254 DOI: 10.1007/s00394-010-0166-z Medline
Chen XL, Gong LZ, Xu JX. Antioxidative activity and protective effect of probiotics against high-fat diet-induced sperm damage in rats. Animal. 2013;7:287-92. PMID: 23031185 DOI: 10.1017/S1751731112001528 Medline
Chen YP, Hsiao PJ, Hong WS, Dai TY, Chen MJ. Lactobacillus kefiranofaciens M1 isolated from milk kefir grains ameliorates experimental colitis in vitro and in vivo. J Dairy Sci. 2012;95:63-74. PMID: 22192184 DOI: 10.3168/jds.2011-4696 Medline
Costa J, Braga PC, Rebelo I, Oliveira PF, Alves MG. Mitochondria Quality Control and Male Fertility. Biology (Basel). 2023;12:827. PMID: 37372112 DOI: 10.3390/biology12060827 Medline
Damián MR, Cortes-Perez NG, Quintana ET, Ortiz-Moreno A, Garfias Noguez C, Cruceño-Casarrubias CE, Sánchez Pardo ME, Bermúdez-Humarán LG. Functional Foods, Nutraceuticals and Probiotics: A Focus on Human Health. Microorganisms. 2022;10:1065. PMID: 35630507 DOI: 10.3390/microorganisms10051065 Medline
Danis RB, Samplaski MK. Sperm Morphology: History, Challenges, and Impact on Natural and Assisted Fertility. Curr Urol Rep. 2019;20:43. PMID: 31203470 DOI: 10.1007/s11934-019-0911-7 Medline
Darbandi M, Darbandi S, Agarwal A, Sengupta P, Durairajanayagam D, Henkel R, Sadeghi MR. Reactive oxygen species and male reproductive hormones. Reprod Biol Endocrinol. 2018;16:87. PMID: 30205828 DOI: 10.1186/s12958-018-0406-2 Medline
de Lamirande E, Gagnon C. Reactive oxygen species and human spermatozoa. I. Effects on the motility of intact spermatozoa and on sperm axonemes. J Androl. 1992;13:368-78. PMID: 1331006 DOI: 10.1002/j.1939-4640.1992.tb03327.x Medline
De Marco S, Sichetti M, Muradyan D, Piccioni M, Traina G, Pagiotti R, Pietrella D. Probiotic Cell-Free Supernatants Exhibited Anti-Inflammatory and Antioxidant Activity on Human Gut Epithelial Cells and Macrophages Stimulated with LPS. Evid Based Complement Alternat Med. 2018;2018:1756308. PMID: 30069221 DOI: 10.1155/2018/1756308 Medline
Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science. 2005;308:1635-8. PMID: 15831718 DOI: 10.1126/science.1110591 Medline
Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol. 2021;19:55-71. PMID: 32887946 DOI: 10.1038/s41579-020-0433-9 Medline
Farahani L, Tharakan T, Yap T, Ramsay JW, Jayasena CN, Minhas S. The semen microbiome and its impact on sperm function and male fertility: A systematic review and meta-analysis. Andrology. 2021;9:115-44. PMID: 32794312 DOI: 10.1111/andr.12886 Medline
Gamidov SI, Ovchinnikov RI, Popova AY. [Double-blind, randomized placebo-controlled study of efficiency and safety of complex acetyl-L-carnitine, L-carnitine fumarate and alpha-lipoic acid (Spermactin Forte) for treatment of male infertility]. Urologiia. 2019;(4):62-8. Russian. PMID: 31535807 DOI: 10.18565/urology.2019.4.61-68 Medline
Gatimel N, Moreau J, Parinaud J, Léandri RD. Sperm morphology: assessment, pathophysiology, clinical relevance, and state of the art in 2017. Andrology. 2017;5:845-62. PMID: 28692759 DOI: 10.1111/andr.12389 Medline
Ghosh S, Whitley CS, Haribabu B, Jala VR. Regulation of Intestinal Barrier Function by Microbial Metabolites. Cell Mol Gastroenterol Hepatol. 2021;11:1463-82. PMID: 33610769 DOI: 10.1016/j.jcmgh.2021.02.007 Medline
Helli B, Kavianpour M, Ghaedi E, Dadfar M, Haghighian HK. Probiotic effects on sperm parameters, oxidative stress index, inflammatory factors and sex hormones in infertile men. Hum Fertil (Camb). 2022;25:499-507. PMID: 32985280 DOI: 10.1080/14647273.2020.1824080 Medline
Hurtado de Catalfo GE, Ranieri-Casilla A, Marra FA, de Alaniz MJ, Marra CA. Oxidative stress biomarkers and hormonal profile in human patients undergoing varicocelectomy. Int J Androl. 2007;30:519-30. PMID: 17573856 DOI: 10.1111/j.1365-2605.2007.00753.x Medline
Kızılay F, Altay B. Evaluation of the effects of antioxidant treatment on sperm parameters and pregnancy rates in infertile patients after varicocelectomy: a randomized controlled trial. Int J Impot Res. 2019;31:424-31. PMID: 30659292 DOI: 10.1038/s41443-018-0109-4 Medline
Kopets R, Kuibida I, Chernyavska I, Cherepanyn V, Mazo R, Fedevych V, Gerasymov S. Dietary supplementation with a novel l-carnitine multi-micronutrient in idiopathic male subfertility involving oligo-, astheno-, teratozoospermia: A randomized clinical study. Andrology. 2020;8:1184-93. PMID: 32330373 DOI: 10.1111/andr.12805 Medline
Krzastek SC, Farhi J, Gray M, Smith RP. Impact of environmental toxin exposure on male fertility potential. Transl Androl Urol. 2020;9:2797-813. PMID: 33457251 doi: 10.21037/tau-20-685. Medline
La Fata G, Weber P, Mohajeri MH. Probiotics and the Gut Immune System: Indirect Regulation. Probiotics Antimicrob Proteins. 2018;10:11-21. PMID: 28861741 DOI: 10.1007/s12602-017-9322-6 Medline
Lewis SE, Sterling ES, Young IS, Thompson W. Comparison of individual antioxidants of sperm and seminal plasma in fertile and infertile men. Fertil Steril. 1997;67:142-7. PMID: 8986699 DOI: 10.1016/S0015-0282(97)81871-7 Medline
Lundy SD, Sangwan N, Parekh NV, Selvam MKP, Gupta S, McCaffrey P, Bessoff K, Vala A, Agarwal A, Sabanegh ES, Vij SC, Eng C. Functional and Taxonomic Dysbiosis of the Gut, Urine, and Semen Microbiomes in Male Infertility. Eur Urol. 2021;79:826-36. PMID: 33573862 DOI: 10.1016/j.eururo.2021.01.014 Medline
Macklaim JM, Clemente JC, Knight R, Gloor GB, Reid G. Changes in vaginal microbiota following antimicrobial and probiotic therapy. Microb Ecol Health Dis. 2015;26:27799. PMID: 26282697 DOI: 10.3402/mehd.v26.27799 Medline
Mancini A, Oliva A, Vergani E, Festa R, Silvestrini A. The Dual Role of Oxidants in Male (In)fertility: Every ROSe Has a Thorn. Int J Mol Sci. 2023;24:4994. PMID: 36902424 DOI: 10.3390/ijms24054994 Medline
Mann U, Shiff B, Patel P. Reasons for worldwide decline in male fertility. Curr Opin Urol. 2020;30:296-301. PMID: 32168194 DOI: 10.1097/MOU.0000000000000745 Medline
Maretti C, Cavallini G. The association of a probiotic with a prebiotic (Flortec, Bracco) to improve the quality/quantity of spermatozoa in infertile patients with idiopathic oligoasthenoteratospermia: a pilot study. Andrology. 2017;5:439-44. PMID: 28245352 DOI: 10.1111/andr.12336 Medline
Mashatan N, Heidari R, Altafi M, Amini A, Ommati MM, Hashemzaei M. Probiotics in vaginal health. Pathog Dis. 2023;81:ftad012. PMID: 37286796 DOI: 10.1093/femspd/ftad012 Medline
Mishra V, Shah C, Mokashe N, Chavan R, Yadav H, Prajapati J. Probiotics as potential antioxidants: a systematic review. J Agric Food Chem. 2015;63:3615-26. PMID: 25808285 DOI: 10.1021/jf506326t Medline
Monteiro C, Marques PI, Cavadas B, Damião I, Almeida V, Barros N, Barros A, Carvalho F, Gomes S, Seixas S. Characterization of microbiota in male infertility cases uncovers differences in seminal hyperviscosity and oligoasthenoteratozoospermia possibly correlated with increased prevalence of infectious bacteria. Am J Reprod Immunol. 2018;79:e12838. PMID: 29500854 DOI: 10.1111/aji.12838 Medline
Nanda Kumar NS, Balamurugan R, Jayakanthan K, Pulimood A, Pugazhendhi S, Ramakrishna BS. Probiotic administration alters the gut flora and attenuates colitis in mice administered dextran sodium sulfate. J Gastroenterol Hepatol. 2008;23:1834-9. PMID: 19120873 DOI: 10.1111/j.1440-1746.2008.05723.x Medline
Pizzino G, Irrera N, Cucinotta M, Pallio G, Mannino F, Arcoraci V, Squadrito F, Altavilla D, Bitto A. Oxidative Stress: Harms and Benefits for Human Health. Oxid Med Cell Longev. 2017;2017:8416763. PMID: 28819546 DOI: 10.1155/2017/8416763 Medline
Ray PF, Toure A, Metzler-Guillemain C, Mitchell MJ, Arnoult C, Coutton C. Genetic abnormalities leading to qualitative defects of sperm morphology or function. Clin Genet. 2017;91:217-32. PMID: 27779748 DOI: 10.1111/cge.12905 Medline
Rinninella E, Raoul P, Cintoni M, Franceschi F, Miggiano GAD, Gasbarrini A, Mele MC. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms. 2019;7:14. PMID: 30634578 DOI: 10.3390/microorganisms7010014 Medline
Slifer ZM, Blikslager AT. The Integral Role of Tight Junction Proteins in the Repair of Injured Intestinal Epithelium. Int J Mol Sci. 2020;21:972. DOI: 10.3390/ijms21030972 PMID: 32024112 DOI: 10.3390/ijms21030972 Medline
Subramanian V, Ravichandran A, Thiagarajan N, Govindarajan M, Dhandayuthapani S, Suresh S. Seminal reactive oxygen species and total antioxidant capacity: Correlations with sperm parameters and impact on male infertility. Clin Exp Reprod Med. 2018;45:88-93. PMID: 29984209 DOI: 10.5653/cerm.2018.45.2.88 Medline
Sumner RN, Tomlinson M, Craigon J, England GCW, Lea RG. Independent and combined effects of diethylhexyl phthalate and polychlorinated biphenyl 153 on sperm quality in the human and dog. Sci Rep. 2019;9:3409. DOI: 10.1038/s41598-019-39913-9 PMID: 30833626 DOI: 10.1038/s41598-019-39913-9 Medline
Valcarce DG, Genovés S, Riesco MF, Martorell P, Herráez MP, Ramón D, Robles V. Probiotic administration improves sperm quality in asthenozoospermic human donors. Benef Microbes. 2017;8:193-206. PMID: 28343402 DOI: 10.3920/BM2016.0122 Medline
Venneri MA, Franceschini E, Sciarra F, Rosato E, D’Ettorre G, Lenzi A. Human genital tracts microbiota: dysbiosis crucial for infertility. J Endocrinol Invest. 2022;45:1151-60. PMID: 35113404 DOI: 10.1007/s40618-022-01752-3 Medline
Wang H, Xu A, Gong L, Chen Z, Zhang B, Li X. The Microbiome, an Important Factor That Is Easily Overlooked in Male Infertility. Front Microbiol. 2022;13:831272. PMID: 35308385 DOI: 10.3389/fmicb.2022.831272 Medline
Wang Y, Wu Y, Wang Y, Xu H, Mei X, Yu D, Wang Y, Li W. Antioxidant Properties of Probiotic Bacteria. Nutrients. 2017;9:521. PMID: 28534820 DOI: 10.3390/nu9050521 Medline
Weng SL, Chiu CM, Lin FM, Huang WC, Liang C, Yang T, Yang TL, Liu CY, Wu WY, Chang YA, Chang TH, Huang HD. Bacterial communities in semen from men of infertile couples: metagenomic sequencing reveals relationships of seminal microbiota to semen quality. PLoS One. 2014;9:e110152. PMID: 25340531 DOI: 10.1371/journal.pone.0110152 Medline