JBRA Assist. Reprod. 2021;25(1):131-135
ORIGINAL ARTICLE
doi: 10.5935/1518-0557.20200064
Randomized controlled trial comparing embryonic quality in rFSH versus hMG in the IVF protocol with GnRH Antagonist
1Universidade Federal do Rio Grande do Sul, Programa de Pós-Graduação em Medicina: Ginecologia e Obstetrícia. Obstetrics and Gynecology Department, Porto Alegre, RS, Brazil
2Hospital de Clínicas de Porto Alegre, Universidade Federal do Rio Grande do Sul, Obstetrics and Gynecology Department, Porto Alegre, RS, Brazil
CONFLICT OF INTEREST
The author has no conflict of interest to declare.
Trial registration date: 13-0120 (IRB-equivalent) and NCT02412904 (Clinical Trials protocol registration system).
ABSTRACT
Objective: The aim of the present study is to investigate embryo quality (score) after
controlled ovarian stimulation for IVF using rFSH or hMG with the GnRH
antagonist protocol.
Methods: Open, randomized, single center study. The patients were randomized to
receive rFSH or hMG according to randomized cards inside a black envelope
with the name of the respective treatment following a computer generated
list (85 patients were allocated to rFSH group and 83 patients to hMG
group). Inclusion criteria were patients with IVF indication and normal
ovarian reserve. Embryo evaluation was performed on day three, after
fertilization based on the Graduated Embryo Score (GES).
Results: There were no relevant differences in demographic characteristics. There was
no difference in pregnancy rates with 27 (31%) and 25 (30.1%) pregnancies
for rFSH and hMG, respectively (p=0.87). The total embryo
score was the same for both groups, but the best embryo score was
significant higher for the rFSH group (77.33±34.0 x 65.07±33.2 p=0.03). The total number of embryos was statistical
different, also in favor of the rFSH group (4.17±3.1 x
3.26±2.4 p=0.04).
Conclusion: The total embryo score was the same for both groups, but the best embryo
score was significantly higher for the rFSH group. Moreover, rFSH was
associated with an increased number of embryos.
Keywords: In vitro fertilization, rFSH, hMG, GnRH antagonist, embryonic quality
INTRODUCTION
We still do not completely understand the specific action of each gonadotropin in the
folliculogenesis. The difficulty to understand their differences is even greater
when we consider the heterogeneity of studies regarding the design, group of
patients and type of protocol used to prevent the ovulation (GnRH agonist or GnRH
antagonist). Because the GnRH agonist was the first analog to be used in IVF cycles,
there are more studies with this protocol. In recent years the GnRH antagonist has
been even more utilized in IVF protocols, but there is a limited number of studies
comparing recombinant follicle stimulating hormone (rFSH) and human menopause
gonadotropin (hMG) when this protocol is used (Karlström et al. , 2018; Olivennes et al., 2002).
Some studies have demonstrated differences in clinical IVF outcomes, such as number
of oocytes retrieved and number or quality of embryos when comparing rFSH and hMG to
ovarian stimulation. A correlation between the number of oocytes retrieved (Bosch et al., 2008) and a
higher number of embryos (Bjercke et
al., 2010) when using the rFSH have already been detected in
some studies, but without detectable difference in pregnancy rates.
Other studies showed opposite results and better outcomes with hMG. In 2008 a
meta-analysis compared hMG and rFSH in the long GnRH agonist protocol. They analyzed
seven randomized trials, including 2259 IVF cycles, and found a significant increase
in life birth rates with hMG when compared to rFSH, with a relative risk of 1.18
(Coomarasamy et al.,
2008). None of these seven trials individually showed a statistically
significant benefit towards hMG, although five of them showed a trend in favor of
hMG.
Besides the lower number and heterogeneity of studies analyzing the GnRH antagonist
protocol, we see that most of the studies are not well designed. Most of these
studies used pregnancy rates as the main outcome, even when they do not enroll a
significant number of patients to find statistical differences in the results. Thus
resulting in an underpowered study, since the number of patients needed to find
statistical differences in these outcomes would be 2,400, and we did not find this
in our literature review. Hence, the few studies that enrolled a higher number of
patients declare themselves sponsored by the pharmaceutical industry (Andersen et al., 2006).
Since embryonic quality is considered to be in direct correlation with pregnancy
rates, we chose to set it as our main outcome. Few studies have compared this
relevant predictor of IVF success. In addition, the number of patients needed to
achieve a high statistical power is lower than the number needed to find differences
when comparing pregnancy rates, which makes it suitable for our research center.
Some authors demonstrated that blastulation could be linked to hMG administration,
although the mechanism was not clear (Ziebe et al., 2007).
Considering the controversial results until now and the lack of knowledge in this
important field of reproductive techniques, we aimed to better understand the
differences in ovarian stimulation comparing embryonic quality with rFSH and hMG in
IVF cycles with GnRH antagonist protocol.
MATERIALS AND METHODS
Design
This was a randomized, open-label, single-center controlled study to compare hMG
(Menopur®, Ferring Pharmaceuticals, Denmark) and rFSH
(Puregon®, Organon Ltd., Ireland) in patients undergoing
ovarian stimulation for IVF using the GnRH antagonist protocol. Our patients
were randomized 1:1 to receive rFSH or hMG according to randomized cards inside
a black envelope with the name of the respective treatment following a
computer-generated list. The present study was included in the Clinical Trials
protocol registration system - NCT02412904. We used the CONSORT statement for
RCT.
Patients and Sample Size Estimation
Infertile patients from a single center of reproductive medicine with indication
for IVF were randomized to receive hMG or rFSH for ovarian stimulation. The
patients were invited to participate if they met all the following criteria of
normal ovarian reserve: normal FSH <10 mUI/ml; Anti Müllerian Hormone (AMH)
between 1 and 3 ng/ml; AFC (Antral Follicle Count) >12; and regular menses
(25-35 days).
Patients were excluded if they had endocrine pathologies, severe masculine factor
(all patients were submitted to IVF without ICSI), previous pelvic surgery or
ovarian cysts.
We estimated the sample size using a significance level of 0.05 and a power of
80% to detect a relevant difference in the embryonic quality between groups,
based on a previous study (Bosch et
al., 2008).
The study started after the approval from the Ethical Committee. All patients
were informed that the study would not interfere or present any risks for their
treatment.
Intervention and Protocol
All the patients had their ovarian reserve analyzed (AMH and AFC), and an
ultrasound scan performed to rule out ovarian cysts and other pelvic
abnormalities prior to treatment. After the complete evaluation, the patients
were asked to schedule an ultrasound scan at the beginning of the menstrual
cycle (first three days). At this time, we started ovarian stimulation with rFSH
or hMG (according to previous randomization), using a dose between 150-300IU
according to their AMH and AFC. This dose was maintained until day 6 of
stimulation, when a second ultrasound scan was performed and the GnRH antagonist
(0.25mg Ganirelix, Orgalutran® Merck Sharp & Dohme,
Australia) was initiated and continued until the end of the cycle. Seriated
ultrasound scans were performed every other day and the hCG (human chorionic
gonadotropin, 5,000 IU Choriomon® IBSA Institut Biochimique
S.A., Switzerland) was administered when at least three follicles reached 17 mm
of mean diameter. We retrieved oocytes 36 hours afterwards.
We assessed the embryos on day three after fertilization, based on the Graduated
Embryo Score (GES) (Fisch et
al., 2001). Three evaluations were performed at 16-18 hours,
25-27 hours and 64-67 hours post fertilization, respectively, by the same
embryologist who was blinded for the intervention. The score was composed by the
following criteria: nucleolar alignment along pronuclear axis, regular cleavage
and degree of fragmentation at the first cell division, and cell number and
morphology on day 3 after fertilization. The maximum score was 100. The total
score was calculated by the sum of embryo scores. The embryos were transferred
on day 3. The patients were advised to have a pregnancy test done 12 days after
the embryo transfer.
All outcomes (dose of gonadotropins, number and size of follicles, number and
score of embryos) were registered during cycles by a restricted and trained team
of three doctors and two embryologists.
This study was not sponsored by the pharmaceutical industry.
Objective
To compare embryonic quality and other clinical outcomes in IVF cycles with GnRH
antagonist protocol using human menopause gonadotropin (hMG) or recombinant
follicle stimulating hormone (rFSH).
Our primary outcome was embryonic quality, based on total embryo score and best
embryo score. The embryo evaluation was performed based on the Graduated Embryo
Score. Moreover, the secondary outcomes were total dose of gonadotropins, number
and size of follicles at the end of the ovarian stimulation, number of retrieved
metaphase II oocytes and clinical pregnancy rates.
Statistical Analysis
We ran the statistical analysis using the SPSS 20® software,
applying the Student T-test for independent samples and the Levene test for
equality of variances. Data are expressed as mean ± standard deviation
(SD) for continuous variables, and as mean and 95% confidence interval (95%CI)
for categorical values. We ran a multivariable analysis to investigate
confounding factors.
RESULTS
A total of 265 patients were eligible (flowchart), 97 were excluded because they did
not fulfill the inclusion criteria or they refused to participate in the study. One
hundred and sixty eight patients were randomized, 85 patients were allocated to the
rFSH group and 83 patients to the hMG group (Figure
1). There were no relevant differences in the demographic characteristics
including mean age, body mass index (BMI) and ovarian reserve tests (Table 1).
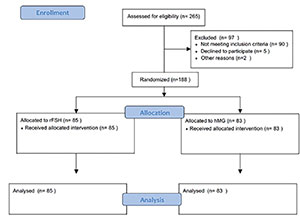
Figure 1. CONSORT Flow Diagram
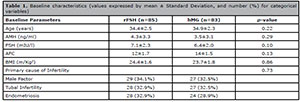
Table 1. Baseline characteristics (values expressed by mean ± Standard Deviation, and number (%) for categorical
variables)
The major causes of infertility were tubal factor (35.7%), masculine factor (33.3%)
and endometriosis (30.9%), without differences in the distribution between the
studied groups.
All the patients had at least one embryo transferred on day 3. The maximum number of
embryos to be transferred was decided in accordance to patients’ desire and age.
Patients under 30 years had only 1 embryo transferred, 31 to 35 years 1 to 2, over
36 years 1 to 3.
The total embryo score was the same for both groups but the best embryo score was
significantly higher for the rFSH group (77.33±34.0 x 65.07±33.2, p=0.03). The total number of embryos was statistical different,
also in favor of the rFSH group (4.17±3.1 x 3.26±2.4, p=0.04).
Table 2 shows the ovarian stimulation
outcomes. Differences in pregnancy rates were not seen among these 27 (31%) and 25
(30.1%) pregnancies for the rFSH and the hMG respectively (p=0.87).
Considering the other secondary outcomes, the total dose of administered
gonadotropins, number of MII oocytes and size of follicles, no statistical
difference was detected.
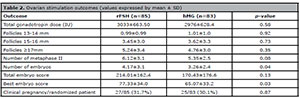
Table 2. Ovarian stimulation outcomes (values expressed by mean ± SD)
The logistic regression model also confirmed that the number of embryos and the best embryo score (day-3) were linked to rFSH administration during controlled ovarian stimulation.
DISCUSSION
Many studies have compared rFSH and hMG in IVF cycles regarding their effectiveness
in ovarian stimulation. Since hMG has a different composition, concerning the
presence of LH, it has been speculated that this would affect the outcomes in
follicular recruitment, follicular growth, number and quality of embryos and
pregnancy rates (Bordewijket al.,
2019; Zeleznik & Kubik, 1986; Ziebeet al., 2007).
There are few studies comparing rFSH and hMG for IVF with the GnRH antagonist
protocol. Our group found some statistical differences in the pattern of ovarian
stimulation of these two gonadotropins. There was a higher number of embryos, and a
higher score of embryo quality in the rFSH group, despite the fact that no
difference was detected in the number of retrieved MII oocytes. This could reflect a
role of the LH activity in the follicular phase that may cause some negative impact
on oocyte quality. We know that the LH/hCG is involved in the process of oocyte
atresia (apoptosis), so this mechanism could also interfere in oocyte quality and
thereafter in embryo quality (Hirata et
al., 2015).
Our findings are in accordance to previous clinical randomized trials that compared
rFSH and hMG in GnRH antagonist cycles (Bordewijket al., 2019; Bosch et al., 2008). They observed a lower
number of oocytes retrieved with a mean difference of 3.1 in favor of the hMG group,
and a lower number of MII oocytes with a mean difference of 1.9. The lower number of
retrieved oocytes in hMG group was also explained by the LH effect during follicular
phase, and its involvement in the atresia process. They found no differences in
pregnancy rates.
These findings also coincide with studies that included the GnRH agonist protocol,
including the Merit study (Ziebe et
al., 2007) which showed a significantly higher number of
oocytes retrieved in the rFSH group. Despite the lower number of oocytes in the hMG
group, and differently from our findings, they detected the best embryonic quality
in this group. The influence of the long agonist protocol, that causes a more
intense pituitary suppression than the GnRH antagonist, could have impacted this
controversial result. Despite these differences, pregnancy rates did not differ
between rFSH or hMG, which was confirmed recently in a meta-analysis (Bordewijk et al., 2019).
Our study found similar pregnancy rates for both groups, which is in accordance with
the few studies that have included this specific IVF protocol (Bosch et al., 2008; Devroey et al., 2012). Although the efficacy of
both drugs did not differ, we found some particularities in their profile of ovarian
stimulation that need to be better understood and will be further discussed.
The variability of IVF protocols and patients’ profiles has complicated the studies
in this field. Most of these studies included patients that used the GnRH agonist
protocol, mainly the long protocol (Andersen et al., 2006; Bjercke et al., 2010; Olivennes et al., 2002). The results are very
controversial in terms of hormonal profile during ovarian stimulation with some
differences in follicular recruitment and embryonic quality. Platteau et al. (2004) analyzed 727 IVF cycles
with the GnRH agonist protocol and found more oocytes retrieved in the rFSH group,
despite a significant more positive beta-hCG test in the hMG group of IVF patients.
This result was not seen in the subgroup analysis of the patients submitted to ICSI.
They speculate that the LH activity could have a beneficial effect on the pregnancy
rates in women undergoing IVF, when the female factor is the main cause of
infertility.
Our study was carried out in a single center with a restricted number of researchers,
which has the advantage of avoiding potential confounding factors and some biases
such as dose adjustment policy, ultrasound measures and embryo assessment criteria.
We intended to minimize the bias of the hormonal profile of the patients considering
the influence of the response to exogenous gonadotropins, so we included only women
with regular menses, normal ovarian reserve tests and no endocrine diseases.
There is a lack of information whether or not the LH activity in ovarian stimulation
preparations improves the outcomes in IVF. Is it beneficial for some specific
population? A Cochrane systematic review in 2007 analyzed 14 clinical trials (eleven
of them using the GnRH agonist); including 2,612 patients and they compared rFSH versus rFSH plus recombinant LH (rLH). There was no statistical
difference in pregnancy rates, but three trials, that included only poor responders,
showed significant increases in pregnancy rates, favoring the co-administration of
rLH, and these results were confirmed more recently by others authors (Minareci & Ozcan, 2019; Mochtar et al., 2007; Shahrokh Tehraninejad et al.,
2017).
In conclusion, we had results that statistically differed in the number of embryos
and the best embryonic score in favor of the rFSH group. We suppose that
gonadotropins might have some impact in oocyte and embryo quality, maybe because of
some interference of the LH/hCG presence in hMG preparations. Further studies are
needed to better explain these findings.
ACKNOWLEDGEMENT
The authors thank the team of Insemine and Projeto
Cegonha for all the support given to carry out this study.
REFERENCES
Andersen AN, Devroey P, Arce JC. Clinical outcome following
stimulation with highly purified hMG or recombinant FSH in patients undergoing
IVF: a randomized assessor-blind controlled trial. Hum Reprod. 2006;21:3217-27.
Medline Crossref
Bjercke S, Tanbo T, Abyholm T, Omland A, Opøien HK, Fedorcsak
P. Clinical outcome following stimulation with highly purified hMG or
recombinant FSH in patients undergoing their first treatment cycle of IVF or
ICSI. Acta Obstet Gynecol Scand. 2010;89:1053-60.
Medline Crossref
Bordewijk EM, Mol F, van der Veen F, Van Wely M. Required amount of
rFSH, HP-hMG and HP-FSH to reach a live birth: a systematic review and
meta-analysis. Hum Reprod Open. 2019;2019:hoz008.
Crossref
Bosch E, Vidal C, Labarta E, Simon C, Remohi J, Pellicer A. Highly
purified hMG versus recombinant FSH in ovarian hyperstimulation with GnRH
antagonists--a randomized study. Hum Reprod. 2008;23:2346-51.
Medline Crossref
Coomarasamy A, Afnan M, Cheema D, van der Veen F, Bossuyt PM, van
Wely M. Urinary hMG versus recombinant FSH for controlled ovarian
hyperstimulation following an agonist long down-regulation protocol in IVF or
ICSI treatment: a systematic review and meta-analysis. Hum Reprod.
2008;23:310-5.
Medline Crossref
Devroey P, Pellicer A, Nyboe Andersen A, Arce JC; Menopur in GnRH
Antagonist Cycles with Single Embryo Transfer Trial Group. A randomized
assessor-blind trial comparing highly purified hMG and recombinant FSH in a GnRH
antagonist cycle with compulsory single-blastocyst transfer. Fertil Steril.
2012;97:561-71.
Medline Crossref
Fisch JD, Rodriguez H, Ross R, Overby G, Sher G. The Graduated
Embryo Score (GES) predicts blastocyst formation and pregnancy rate from
cleavage-stage embryos. Hum Reprod. 2001;16:1970-5.
Medline Crossref
Hirata R, Hojo T, Sano M, Hayashi N, Okuda K. Potential role of hCG
in apoptosis of human luteinized granulosa cells. J Reprod Dev. 2015;61:67-73.
Medline Crossref
Karlström PO, Holte J, Hadziosmanovic N, Rodriguez-Wallberg KA,
Olofsson JI. Does ovarian stimulation regimen affect IVF outcome? a two-centre,
real-world retrospective study using predominantly cleavage-stage, single embryo
transfer. Reprod Biomed Online. 2018;36:59-66.
Medline Crossref
Mochtar MH, Van der Veen, Ziech M, van Wely M. Recombinant
Luteinizing Hormone (rLH) for controlled ovarian hyperstimulation in assisted
reproductive cycles. Cochrane Database Syst Rev. 2007:(2)CD005070.
Medline Crossref
Olivennes F, Cunha-Filho JS, Fanchin R, Bouchard P, Frydman R. The
use of GnRH antagonists in ovarian stimulation. Hum Reprod Update.
2002;8:279-90.
Medline Crossref
Platteau P, Smitz J, Albano C, Sørensen P, Arce JC , Devroey
P. Exogenous luteinizing hormone activity may influence the treatment outcome in
in vitro fertilization but not in intracytoplasmic sperm injection cycles.
Fertil Steril. 2004;81:1401-4.
Medline Crossref
Shahrokh Tehraninejad E, Farshbaf Taghinejad M, Hossein Rashidi B,
Haghollahi F. Controlled ovarian stimulation with r-FSH plus r-LH vs. HMG plus
r-FSH in patients candidate for IVF/ICSI cycles: An RCT. Int J Reprod Biomed.
2017;15:435-40.
Medline Crossref
Zeleznik AJ, Kubik CJ. Ovarian responses in macaques to pulsatile
infusion of follicle-stimulating hormone (FSH) and luteinizing hormone:
increased sensitivity of the maturing follicle to FSH. Endocrinology.
1986;119:2025-32.
Medline Crossref
Ziebe S, Lundin K, Janssens R, Helmgaard L, Arce JC; MERIT
(Menotrophin vs Recombinant FSH in vitro Fertilisation Trial) Group. Influence
of ovarian stimulation with HP-hMG or recombinant FSH on embryo quality
parameters in patients undergoing IVF. Hum Reprod. 2007;22:2404-13.
Medline Crossref