JBRA Assist. Reprod. 2023;27(3):428-435
ORIGINAL ARTICLE
doi: 10.5935/1518-0557.20220074
Involvement of SYCP2L and TDRD3 gene variants on ovarian reserve and reproductive outcomes: a cross-sectional study
1Discipline of Sexual and Reproductive Health and Populational Genetics, Department of Collective Health, Faculdade de Medicina do ABC/Centro Universitário Saúde ABC, FMABC, Santo André, São Paulo, Brazil
2Instituto Ideia Fértil, Santo André, Brazil
3Unit of Gynecologic Oncology, ARNAS “Civico - Di Cristina - Benfratelli”, Department of Health Promotion, Mother and Child Care, Internal Medicine and Medical Specialties (PROMISE), University of Palermo, Palermo, Italy
CONFLICTS OF INTEREST
The authors have no conflict of interest to declare.
ABSTRACT
Objective: Single nucleotide variants have been implicated in the response to fertility treatment and a pharmacogenomic approach may help to customize therapy based on patient genome. We aimed to evaluate the effect, individual and combined, of SYCP2L (rs2153157:G>A) and TDRD3 (rs4886238:G>A) variants on ovarian reserve, response to controlled ovarian stimulation (COS) and reproductive outcomes of women undergoing in vitro fertilization (IVF) treatment.
Methods: This cross-sectional study included 149 normoovulatory women undergoing IVF. Genotyping was performed using the TaqMan real-time polymerase chain reaction method. Clinical parameters and reproductive outcomes were compared according to the genotypes of the variants studied.
Results: Considering ovarian reserve, there were no significant differences among SYCP2L or TDRD3 genotypes in terms of FSH levels or AFC; however, AMH levels were significantly different in carriers of both variants. Regarding the SYCP2L rs2153157:G>A variant, lower AMH levels were observed in women carrying an AA genotype compared to women carrying a heterozygous genotype (p=0.01). Considering the TDRD3 rs4886238:G>A variant, women carrying an AA genotype presented higher AMH levels than carriers of GG and GA genotypes (p=0.025). Nevertheless, no difference was found regarding response to COS or reproductive outcomes. Considering the combined effect of the variants, women carrying the heterozygous genotype of both variants presented statistically increased AMH levels compared to SYCP2L rs2153157 AA genotype carriers and TDRD3 rs4886238 GG genotype carriers (p=0.042).
Conclusions: Individually and combined, the SYCP2L rs2153157 and TDRD3 rs4886238 variants have an effect on AMH level.
Keywords: infertility, in vitro fertilization, single nucleotide variant, pharmacogenetic, ovarian reserve
INTRODUCTION
It has been estimated that over 1.5 million in-vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI) cycles are performed annually worldwide, and this number increases each year (Dyer et al., 2016). The outcome of assisted reproduction is largely influenced by the effectiveness of controlled ovarian stimulation (COS) as well as ovarian reserve; however, the response to COS varies widely from woman to woman (Oehninger, 2011; Roque et al., 2019).
The ovarian reserve declines differently in each woman. Besides, the ovarian reserve tests used to date have only modest-to-poor predictive properties (Broekmans et al., 2006; Broer et al., 2013; Amanvermez & Tosun, 2016; Peluso et al., 2021). COS is the first step in every IVF/ICSI cycle and aims to allow the development and maturation of multiple follicles and oocytes, thus increasing cumulative pregnancy rates from IVF. However, some patients present an unexpected hyporesponse or even a hyper-response to gonadotropin stimulation, which can cause adverse events leading to physical and psychological distress (Roque et al., 2019). Consequently, research has been conducted to find reliable predictors of effective COS and good ART outcomes.
Individual genetic variability is known to affect ovarian reserve and the outcome of COS, and a pharmacogenomic approach may help to customize therapy based on patient genome (Roque et al., 2019; Trevisan et al., 2019). Variants in different genes, such as single nucleotide polymorphisms (SNP) or single nucleotide variants (SNV), have been implicated in the response to fertility treatment.
The most studied SNPs are FSHR:c.919G>A, FSHR:c.2039G>A and FSHR:c.-29G>A, which were previously associated with variability in serum FSH level and reproductive outcomes (Pabalan et al., 2014; Alviggi et al., 2016; Santi et al., 2018). Nonetheless, many other gene variants have been studied and appear to influence the IVF outcomes. Laisk-Podar et al. (2015) searched for genetic markers of ovarian function, ovarian stimulation and IVF treatment outcomes among genetic variants related to female reproductive ageing in Estonian patients. The results revealed that among 36 variants searched, the rs2153157 of the SYCP2L gene was associated with amount of recombinant FSH (rFSH) and chance of biochemical and clinical pregnancy. In addition, rs4886238 of the TDRD3 gene was associated with both the number of punctured ovarian follicles and oocytes retrieved.
In this scenario, the aim of this study was to analyze the individual and combined effects of SYCP2L (rs2153157) and TDRD3 (rs4886238) variants on ovarian reserve, response to COS, and reproductive outcomes of Brazilian women undergoing IVF treatment.
MATERIAL AND METHODS
Patients
A cross-sectional study was performed between September 2016 and September 2019 and included 149 normoovulatory Brazilian women undergoing IVF treatment at the Instituto Ideia Fertil - Human Reproduction and Genetics Center of the School of Medicine of the ABC, Santo Andre, Brazil. The Research Ethics Committee of the School of Medicine of the ABC approved the study design (certificate CAAE 64167716.9.1001.0082) and all participants signed informed consent terms before joining the study to allow anonymized data collection for purposes of research.The investigation into the cause of infertility included hormonal and biochemical profiling, testing for sexually transmitted diseases, imaging examinations, investigation of genetic and/or immunological abnormalities, hysterosalpingography, hysteroscopy, laparoscopy, and partner semen analysis.The inclusion criteria were as follows: age ≤38 years old; FSH ≤12.0 IU/L; TSH >0.5 to <4 IU/L; serum prolactin <25 ng/mL; body mass index (BMI) >18.5 to ≤30; ovulatory cycles during 25-35 days; presence of both ovaries without morphological abnormalities; and having no evidence of endocrine disease. Patients with endometriosis, polycystic ovarian syndrome, previous ovarian surgery or chemo/radiotherapy, and couples whose male partner underwent invasive procedures for sperm retrieval were excluded.
Antral follicle count
Ovaries were evaluated before initiation of COS on the second day of the menstrual cycle by transvaginal ultrasonography using a conventional two-dimensional transvaginal ultrasound at 7MHz (Philips®). The antral follicle count (AFC) was performed on each ovary and was considered as follicles counted up to 2-10 mm (Broekmans et al., 2010).
Sample collection
Peripheral blood samples of 15 mL were collected in a tube containing clot-separator gel and in a tube containing ethylenediaminetetraacetic acid (EDTA). After collection, the tubes for biochemical tests were centrifuged at 1000 rpm for 10 min; plasma was aliquoted into microtubes and frozen at -80°C for further determination of hormone concentrations. The tube for DNA extraction was stored at 6°-8°C until extraction.
Hormone level measurements
Follicle-stimulating hormone (FSH), luteinizing hormone (LH), and anti-Müllerian hormone (AMH) levels were measured during the early follicular phase of the menstrual cycle. Progesterone and prolactin were measured during the luteal phase of the menstrual cycle. FSH, LH, progesterone, and prolactin were measured with an enzyme-linked fluorescent immunoassay (BioMerieux®, Hazelwood, MO) and AMH Gen II was measured with an enzyme-linked immunosorbent assay (Beckman Coulter®, Inc., Brea, CA).
IVF treatment
COS was performed using exogenous recombinant FSH (rFSH, Puregon®) at a fixed dose per day administered on average for 8 to 14 days, starting on the second or third day of the menstrual cycle. GnRH antagonist (Orgalutran®) was administered when the follicles reached a diameter of approximately 14 mm, until the largest follicles reached between 17 and 20 mm, as measured in transvaginal ultrasonography. At this time, the patient was given chorionic gonadotropin (hCG-Choriomon®) at a dose of 5000 IU or recombinant HCG (Ovidrel®, Merck S/A, 250 mg/0,5 mL). After 34-36 h, transvaginal ultrasound-guided follicular puncture for oocyte retrieval was performed (Barbosa et al., 2014).The classification of the response to COS was as follows: i) satisfactory response characterized by the development of ≥4 to ≤15 follicles larger than 14 mm after 6 days of ovarian stimulation with gonadotropins; ii) hyper response and/or ovarian hyperstimulation syndrome (OHSS) characterized by multiple ovarian follicles (>15 follicles) together with potential clinical symptoms, such as ascites, hematological changes (hemoconcentration), pleural effusion, and liver and/or coagulation abnormalities; and iii) poor response characterized by the development of up to three follicles smaller than 14mm (Polyzos & Sunkara, 2015).A maximum of two embryos were transferred on the third or fifth day after IVF/ICSI. Luteal phase support was carried out with vaginal progesterone at a dose of 600 mg/day starting on the day of oocyte retrieval. Pregnancy was confirmed by b-hCG measurement (>25 mIU/mL) on Day 12 after embryo transfer.
Genotyping
Genomic DNA was extracted from lymphocytes using the salting out method (Lahiri & Numberger, 1991). Genotyping of the variants SYCP2L:g.10897255G>A (chr6:10897255, NC_000006.12:g.10897255G>A, rs2153157) and TDRD3:g.60539605G>A (chr13:60539605, NC_000013.11:g.60539605G>A, rs4886238) was performed using the TaqMan system by real-time polymerase chain reaction according to manufacturer instructions (ThermoFisher Scientific®, Waltham, MA).
Statistical Analyses
Descriptive analyses were performed based on absolute and relative frequencies for categorical variables, and on medians with a 95% confidence interval for quantitative variables. Data distribution was analyzed with the Shapiro-Wilk test. Hardy-Weinberg equilibrium of the variants studied was verified using the Chi-squared test. The Mann-Whitney test was used to analyze the effect of each variant on clinical characteristics, hormone levels and reproductive outcomes. The chi-squared test was used to certify the associations between the variants and the response to COS, rFSH protocol, and pregnancy rate. The Kruskal-Wallis test was used followed by Dunn’s test to analyze the difference in the combined genotype of the rs4886238 and rs2153157 variants in hormonal profile and reproductive outcomes. Statistical analyses were performed with Stata® software (SE 11.0) for Windows and significance was considered at p<0.05.
RESULTS
Of the 149 women, the genotype distribution for SYCP2L rs2153157:G>A variant was 0% (0/149) carriers of wild-type homozygous genotype (GG), 32.9% (49/149) heterozygous (GA) and 67.1% (100/149) carriers of the variant homozygous genotype (AA). The G and A allele were frequent in 16.5% and 83.5% of the women, respectively. Considering the TDRD3 rs4886238:G>A variant, the genotype distribution was 53% (79/149) wild-type homozygous genotype (GG), 39.6% (59/149) heterozygous and 7.4% (11/149) variant homozygous genotype (AA). The wild-type G allele was found in 72.8% of women, while the variant allele G was found in 27.3%. Both SYCP2L rs2153157:G>A and TDRD3 rs4886238:G>A variants were in Hardy-Weinberg equilibrium, p=0.090 and p=0.544, respectively.
The clinical characteristics, hormonal profile and reproductive outcomes of the women studied are shown in Table 1. Of the 149 normoovulatory women undergoing IVF treatment, 65.1% (97/149) had satisfactory response to COS, 21.5% (32/149) had poor response, and 13.4% (20/149) had hyper-response. Eleven of the 20 hyper-responders presented minor symptoms of ovarian hyperstimulation syndrome (OHHS), such as ovarian enlargement, abdominal bloating, and pain (OHSS grade 1), without significant changes in renal or hepatic function until clinical improvement was achieved. Thirty-one patients had no embryo to transfer: 21 had their cycles canceled due to lack of ovarian response or monofollicular response (14.1% of the IVF cycles); nine had no embryo development; and one patient had empty follicle syndrome. Therefore, the pregnancy rate was calculated based on 118 patients undergoing embryo transfer; 39.0% (46/118) of these patients had positive b-HCG test results.
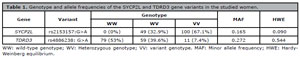
Table 1. Genotype and allele frequencies of the SYCP2L and TDRD3 gene variants in the studied women.
The clinical characteristics, hormonal profile and reproductive outcomes of the women were compared according to SYCP2L (Table 2) and TDRD3 (Table 3) genotypes. Age, BMI, menarche, menstrual cycle interval, and infertility duration did not differ between the genotypes for either SYCP2L or TDRD3 variants. Considering ovarian reserve, there were no significant differences between the SYCP2L or TDRD3 genotypes in terms of FSH levels and AFC. However, AMH levels were significantly different between variants. In the SYCP2L rs2153157:G>A variant, lower AMH levels were observed in women carrying the homozygous variant genotype (AA) compared to women carrying the heterozygous genotype (3.7 ng/mL versus 2.9ng/mL, p=0.01). In the TDRD3 rs4886238:G>A variant, women carrying the homozygous variant genotype (AA) presented higher AMH levels compared to GG and GA genotypes (4.5 ng/mL, 2.8 ng/mL, and 3.4 ng/mL, respectively, p=0.025). Nevertheless, no difference was found in reproductive outcomes.
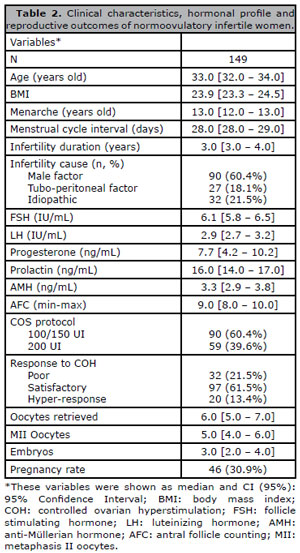
Table 2. Clinical characteristics, hormonal profile and reproductive outcomes of normoovulatory infertile women.
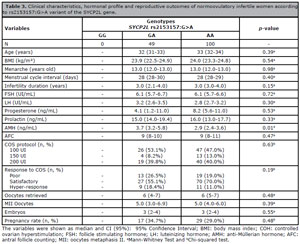
Table 3. Clinical characteristics, hormonal profile and reproductive outcomes of normoovulatory infertile women according to rs2153157:G>A variant of the SYCP2L gene.
Considering the combined effect of the genotypes of the SYCP2L rs2153157:G>A and TDRD3 rs4886238:G>A variants, 41.6% (n=62) of the patients presented the SYCP2L:AA/TDRD3:GG genotypes; 20.1% (n=30) the SYCP2L:GA/TDRD3:AA genotypes; 19.4% (n=29) the SYCP2L:GA/TDRD3:GA genotypes, and 18.8% (n=28) other combinations of genotypes. Only AMH level was different according to combination of genotypes (p=0.031) (Table 4). Women carrying the heterozygous genotype (GA) of both variants presented statistically higher AMH levels compared to women carrying the homozygous variant genotype (AA) of the SYCP2L rs2153157 and wild-type homozygous genotype (GG) of the TDRD3 rs4886238 variant [3.5 (3.0-6.9) ng/mL versus 2.8 (1.9-3.5) ng/mL, p=0.042].
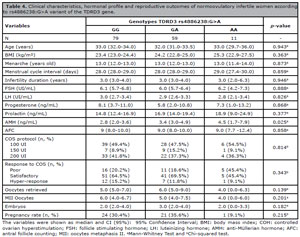
Table 4. Clinical characteristics, hormonal profile and reproductive outcomes of normoovulatory infertile women according to rs4886238:G>A variant of the TDRD3 gene.
DISCUSSION
Despite the growing data about IVF around the world, no biomarker has been described as an accurate predictor of response to COS or IVF reproductive outcome. Use of SNPs that can predict the effectiveness of drugs in individual patients, depending on their genetic background, may add further elements in this direction (Ramaraju et al., 2018).
During oogenesis, homologous chromosomes are paired for recombination between chromosomes, mediated by the formation of a synaptonemic complex, a tripartite structure made up of two lateral/axial elements and a central element (Costa & Cooke, 2007; Yang & Wang, 2009). In rats, the SYCP2 protein is a component of the lateral/axial element (Schalk et al., 1998). In mammals, SYCP2L is a homologous sequence of SYCP2. In Xenopus oocytes, the SYCP2L protein is exclusively expressed in immature oocytes (Voltmer-Irsch et al., 2007), with Sycp2 -/- knockout mice showing accelerated loss of oocytes and reduced fertility, evidencing their role in the regulation of the survival of primordial oocytes (Schramm et al., 2011; Zhou et al., 2015).
The diplotene stage in oocytes extends from birth to ovulation, lasting up to four decades in women. The SYCP2L protein in oocyte centromeres is involved in the organization of the local chromatin around the centromeres and may play an important role in sensing and repairing DNA damage to promote oocyte survival (Zhou et al., 2015). Recent genome-wide association studies (GWAS) showed that variants of the human SYCP2L locus have been associated with age at menopause (He et al., 2009; Carty et al., 2013).
The human SYCP2L gene is located at 6p24.2 and the variant g.10897488G>A (rs2153157) is located at intron 4. Zhou et al. (2015) found that variant allele A changes the splicing efficiency and may therefore regulate the steady-state amount of SYCP2L transcript. The A allele is spliced more efficiently than the G allele, and is thus expressed at a higher level. The authors demonstrated that SYCP2L promotes the survival of reserve oocytes and regulates reproductive aging in females.
The TDRD3 gene (Tudor domain-containing protein 3) is located on chromosome 13q21.2 and is a transcriptional co-activator and regulator of estrogen-mediated gene transcription. In addition, it interacts with the FMRP protein (Fragile X protein) involved in the development of primary ovarian failure (Linder et al., 2008; Sullivan et al., 2011), which suggests a role in ovarian reserve.
In the study of Laisk-Podar et al. (2015), women carrying the AA genotype of the SYCP2L rs2153157:G>A variant needed less rFSH to obtain an oocyte and had greater chances of attaining biochemical and clinical pregnancy. The authors srtressed that these results may indicate that carriers of the AA genotype have greater ovarian reserve. In addition, the authors suggested that the positive effect of the variant allele in clinical pregnancy rates may result directly from having a larger ovarian reserve or be associated with the role that the synaptonemal complex plays in preventing chromosome segregation errors and embryo aneuploidy, one of the main causes of implantation failure and early miscarriage. Regarding the TDRD3 rs4886238:G>A variant, COS resulted in more follicles and oocytes in women carrying the G allele. The authors stressed that previously the G allele had been associated with earlier menopause (Stolk et al., 2012) and this finding along with their results indicate that the ovarian pool is depleted more quickly in women carrying the G allele.
In the present study, none of the included women had the GG genotype of the SYCP2L rs2153157:G>A variant. Although the occurrence the minor G allele was lesser than in different genetic databases (Reference SNP Report - rs2153157, 2021), the frequency of the genotypes was in Hardy-Weinberg equilibrium in the studied population. Unlike Laisk-Podar et al. (2015), in our study the A allele was not associated with having a larger ovarian reserve, since ovarian reserve marker such as age, FSH and AFC were not different between carriers of G or A alleles. The A allele variant was not significantly associated with AMH levels, whereas women with the AA genotype presented significantly lower AMH levels in comparison to women with the GA genotype. Nevertheless, pregnancy rates were not different between alleles. Regarding the TDRD3 rs4886238:G>A variant, women carrying the AA genotype presented statistically higher AMH levels than their counterparts with the GA and GG genotypes (Table 3). However, no differences were observed in ovarian reserve tests or reproductive outcomes. The combined effect of the SYCP2L rs2153157:G>A and the TDRD3 rs4886238:G>A variants was different only to AMH levels, whereas women with the heterozygous genotype (GA) of both variants presented statistically higher AMH levels compared to women with the homozygous variant genotype (AA) of the SYCP2L rs2153157 and wild-type homozygous genotype (GG) of the TDRD3 rs4886238 variant.
The different findings between studies can be attributed to several factors: patient selection criteria; sample size; ovarian stimulation protocol; and differences in ethnicity, all of which hinder the interpretation of the results. The Brazilian population was built with contributions from Europeans, Africans and Amerindians, resulting in a highly heterogenous genetic profile, the likes of which seldom seen in other parts of the world (Gaspar Neto & Santos, 2011; Salzano & Sans, 2014; Ramos et al., 2016). Due to genetic diversity, the Brazilian population may show different allele frequencies than those presented in non-mixed populations. Nevertheless, Brazil is not represented in genomic datasets, such as gnomAD and TOPMed, although these databases have recently included Latin American samples (Naslavsky et al., 2022).
AMH is considered the most sensitive ovarian reserve test (Tal & Seifer, 2017). It is strongly correlated with the primordial follicle pool and has an inversely proportional correlation with age (Kelsey et al., 2012). Revelli et al. (2016) observed that in women with very low AMH levels, the ones who achieved clinical pregnancy had AMH levels comparable to those who did not achieve pregnancy. Peluso et al. (2021) evaluated age, FSH, AMH, AFC, and the ovarian response prediction index (ORPI), as potential predictors of response to COS, and found that none of them individually or combined showed good predictive capacity for hypo-response. The authors also found that AMH alone was the best predictor for hyper-response, while the ORPI demonstrated the best predictive capacity. Indeed, it is still debatable whether AMH might be considered a reliable marker of IVF outcomes (Peñarrubia et al., 2005; Fiçicioglu et al., 2006; Lekamge et al., 2007; Broer et al., 2011; Siddiqui et al., 2019; Peluso et al., 2021).
CONCLUSION
In conclusion, SYCP2L rs2153157 and TDRD3 rs4886238 variants individually have an effect on AMH levels, whereas the homozygous variant genotype AA was associated with lower AMH levels for the SYCP2L rs2153157:G>A variant and higher AMH levels for the TDRD3 rs4886238:G>A variant. No differences were found in other markers of ovarian reserve, response to COS, or reproductive outcomes in the genotypes of the studied variants. The combined effects of the SYCP2L rs2153157 and TDRD3 rs4886238 variants also have an effect on AMH levels, while women carrying the combination of GA genotypes of both variants presented higher AMH levels.
Acknowledgment
The authors would like to thank The São Paulo Research Foundation-FAPESP and The Conselho Nacional de Desenvolvimento Científico e Tecnológico - CNPq for granting, respectively, Caroline Awoki Ferrandez (FAPESP #18/25270-4) and Bianca Del Bel Sonoda a student scholarship.
REFERENCES
Alviggi C, Conforti A, Caprio F, Gizzo S, Noventa M, Strina I, Pagano T, De Rosa P, Carbone F, Colacurci N, De Placido G. In Estimated Good Prognosis Patients Could Unexpected “Hyporesponse” to Controlled Ovarian Stimulation be Related to Genetic Polymorphisms of FSH Receptor? Reprod Sci. 2016;23:1103-8. PMID: 26902430 DOI: 10.1177/1933719116630419 Medline
Amanvermez R, Tosun M. An Update on Ovarian Aging and Ovarian Reserve Tests. Int J Fertil Steril. 2016;9:411-5. PMID: 26985328 DOI: 10.22074/ijfs.2015.4591 Medline
Barbosa CP, Cordts EB, Costa AC, de Oliveira R, de Mendonça MA, Christofolini DM, Bianco B. Low dose of rFSH [100 IU] in controlled ovarian hyperstimulation response: a pilot study. J Ovarian Res. 2014;7:11. PMID: 24447686 DOI: 10.1186/1757-2215-7-11 Medline
Broekmans FJ, de Ziegler D, Howles CM, Gougeon A, Trew G, Olivennes F. The antral follicle count: practical recommendations for better standardization. Fertil Steril. 2010;94:1044-51. PMID: 19589513 DOI: 10.1016/j.fertnstert.2009.04.040 Medline
Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update. 2006;12:685-718. PMID: 16891297 DOI: 10.1093/humupd/dml034 Medline
Broer SL, Dólleman M, Opmeer BC, Fauser BC, Mol BW, Broekmans FJ. AMH and AFC as predictors of excessive response in controlled ovarian hyperstimulation: a meta-analysis. Hum Reprod Update. 2011;17:46-54. PMID: 20667894 DOI: 10.1093/humupd/dmq034 Medline
Broer SL, van Disseldorp J, Broeze KA, Dolleman M, Opmeer BC, Bossuyt P, Eijkemans MJ, Mol BW, Broekmans FJ; IMPORT study group. Added value of ovarian reserve testing on patient characteristics in the prediction of ovarian response and ongoing pregnancy: an individual patient data approach. Hum Reprod Update. 2013;19:26-36. PMID: 23188168 DOI: 10.1093/humupd/dms041 Medline
Carty CL, Spencer KL, Setiawan VW, Fernandez-Rhodes L, Malinowski J, Buyske S, Young A, Jorgensen NW, Cheng I, Carlson CS, Brown-Gentry K, Goodloe R, Park A, Parikh NI, Henderson B, Le Marchand L, Wactawski-Wende J, Fornage M, Matise TC, Hindorff LA, et al. Replication of genetic loci for ages at menarche and menopause in the multi-ethnic Population Architecture using Genomics and Epidemiology (PAGE) study. Hum Reprod. 2013;28:1695-706. PMID: 23508249 DOI: 10.1093/humrep/det071 Medline
Costa Y, Cooke HJ. Dissecting the mammalian synaptonemal complex using targeted mutations. Chromosome Res. 2007;15:579-89. PMID: 17674147 DOI: 10.1007/s10577-007-1142-1 Medline
Dyer S, Chambers GM, de Mouzon J, Nygren KG, Zegers-Hochschild F, Mansour R, Ishihara O, Banker M, Adamson GD. International Committee for Monitoring Assisted Reproductive Technologies world report: Assisted Reproductive Technology 2008, 2009 and 2010. Hum Reprod. 2016;31:1588-609. PMID: 27207175 DOI: 10.1093/humrep/dew082 Medline
Fiçicioglu C, Kutlu T, Baglam E, Bakacak Z. Early follicular antimüllerian hormone as an indicator of ovarian reserve. Fertil Steril. 2006;85:592-6. PMID: 16500324 DOI: 10.1016/j.fertnstert.2005.09.019 Medline
He C, Kraft P, Chen C, Buring JE, Paré G, Hankinson SE, Chanock SJ, Ridker PM, Hunter DJ, Chasman DI. Genome-wide association studies identify loci associated with age at menarche and age at natural menopause. Nat Genet. 2009;41:724-8. PMID: 19448621 DOI: 10.1038/ng.385 Medline
Kelsey TW, Anderson RA, Wright P, Nelson SM, Wallace WH. Data-driven assessment of the human ovarian reserve. Mol Hum Reprod. 2012;18:79-87. PMID: 21933846 DOI: 10.1093/molehr/gar059 Medline
Lahiri DK, Nurnberger JI Jr. A rapid non-enzymatic method for the preparation of HMW DNA from blood for RFLP studies. Nuclei Acids Res. 1991;19:5444. PMID: 1681511 DOI: 10.1093/nar/19.19.5444 Medline
Laisk-Podar T, Kaart T, Peters M, Salumets A. Genetic variants associated with female reproductive ageing--potential markers for assessing ovarian function and ovarian stimulation outcome. Reprod Biomed Online. 2015;31:199-209. DOI: 10.1016/j.rbmo.2015.05.001 PMID: 26099445 DOI: 10.1016/j.rbmo.2015.05.001 Medline
Lekamge DN, Barry M, Kolo M, Lane M, Gilchrist RB, Tremellen KP. Anti-Müllerian hormone as a predictor of IVF outcome. Reprod Biomed Online. 2007;14:602-10. DOI: 10.1016/s1472-6483(10)61053-x PMID: 17509203 DOI: 10.1016/S1472-6483(10)61053-X Medline
Linder B, Plöttner O, Kroiss M, Hartmann E, Laggerbauer B, Meister G, Keidel E, Fischer U. Tdrd3 is a novel stress granule-associated protein interacting with the Fragile-X syndrome protein FMRP. Hum Mol Genet. 2008;17:3236-46. DOI: 10.1093/hmg/ddn219 PMID: 18664458 DOI: 10.1093/hmg/ddn219 Medline
Naslavsky MS, Scliar MO, Yamamoto GL, Wang JYT, Zverinova S, Karp T, Nunes K, Ceroni JRM, de Carvalho DL, da Silva Simões CE, Bozoklian D, Nonaka R, Dos Santos Brito Silva N, da Silva Souza A, de Souza Andrade H, Passos MRS, Castro CFB, Mendes-Junior CT, Mercuri RLV, Miller TLA, et al. Whole-genome sequencing of 1,171 elderly admixed individuals from São Paulo, Brazil. Nat Commun. 2022;13:1004. PMID: 35246524 DOI: 10.1038/s41467-022-28648-3 Medline
Oehninger S. Ovulation induction in IVF. Minerva Ginecol. 2011;63:137-56. PMID: 21508903 Medline
Pabalan N, Trevisan CM, Peluso C, Jarjanazi H, Christofolini DM, Barbosa CP, Bianco B. Evaluating influence of the genotypes in the follicle-stimulating hormone receptor (FSHR) Ser680Asn (rs6166) polymorphism on poor and hyper-responders to ovarian stimulation: a meta-analysis. J Ovarian Res. 2014;7:285. PMID: 25526787 DOI: 10.1186/s13048-014-0122-2 Medline
Peluso C, Oliveira R, Laporta GZ, Christofolini DM, Fonseca FLA, Laganà AS, Barbosa CP, Bianco B. Are ovarian reserve tests reliable in predicting ovarian response? Results from a prospective, cross-sectional, single-center analysis. Gynecol Endocrinol. 2021;37:358-66. PMID: 32613875 DOI: 10.1080/09513590.2020.1786509 Medline
Peñarrubia J, Fábregues F, Manau D, Creus M, Casals G, Casamitjana R, Carmona F, Vanrell JA, Balasch J. Basal and stimulation day 5 anti-Mullerian hormone serum concentrations as predictors of ovarian response and pregnancy in assisted reproductive technology cycles stimulated with gonadotropin-releasing hormone agonist--gonadotropin treatment. Hum Reprod. 2005;20:915-22. PMID: 15665015 DOI: 10.1093/humrep/deh718 Medline
Polyzos NP, Sunkara SK. Sub-optimal responders following controlled ovarian stimulation: an overlooked group? Hum Reprod. 2015;30:2005-8. PMID: 26202582 DOI: 10.1093/humrep/dev149 Medline
Ramaraju GA, Cheemakurthi R, Prathigudupu K, Balabomma KL, Kalagara M, Thota S, Kota M. Role of Lh polymorphisms and r-hLh supplementation in GnRh agonist treated ART cycles: A cross sectional study. Eur J Obstet Gynecol Reprod Biol. 2018;222:119-25. PMID: 29408742 DOI: /10.1016/j.ejogrb.2018.01.025 Medline
Ramos BR, D’Elia MP, Amador MA, Santos NP, Santos SE, da Cruz Castelli E, Witkin SS, Miot HA, Miot LD, da Silva MG. Neither self-reported ethnicity nor declared family origin are reliable indicators of genomic ancestry. Genetica. 2016;144:259-65. PMID: 26984822 DOI: 10.1007/s10709-016-9894-1 Medline
Reference SNP Report - rs2153157; 2021. Available at: https://www.ncbi.nlm.nih.gov/snp/rs2153157
Revelli A, Biasoni V, Gennarelli G, Canosa S, Dalmasso P, Benedetto C. IVF results in patients with very low serum AMH are significantly affected by chronological age. J Assist Reprod Genet. 2016;33:603-9. PMID: 26888025 DOI: 10.1007/s10815-016-0675-7 Medline
Roque M, Bianco B, Christofolini DM, Barchi Cordts E, Vilarino F, Carvalho W, Valle M, Sampaio M, Geber S, Esteves SC, Parente Barbosa C. Pharmacogenetic algorithm for individualized controlled ovarian stimulation in assisted reproductive technology cycles. Panminerva Med. 2019;61:76-81. PMID: 29916218 DOI: 10.23736/S0031-0808.18.03496-1 Medline
Salzano FM, Sans M. Interethnic admixture and the evolution of Latin American populations. Genet Mol Biol. 2014;37:151-70. PMID: 24764751 DOI: 10.1590/S1415-47572014000200003 Medline
Santi D, Potì F, Simoni M, Casarini L. Pharmacogenetics of G-protein-coupled receptors variants: FSH receptor and infertility treatment. Best Pract Res Clin Endocrinol Metab. 2018;32:189-200. PMID: 29678285 DOI: 10.1016/j.beem.2018.01.001 Medline
Schalk JA, Dietrich AJ, Vink AC, Offenberg HH, van Aalderen M, Heyting C. Localization of SCP2 and SCP3 protein molecules within synaptonemal complexes of the rat. Chromosoma. 1998;107:540-8. DOI: 10.1007/s004120050340 PMID: 9933407 DOI: 10.1007/s004120050340 Medline
Schramm S, Fraune J, Naumann R, Hernandez-Hernandez A, Höög C, Cooke HJ, Alsheimer M, Benavente R. A novel mouse synaptonemal complex protein is essential for loading of central element proteins, recombination, and fertility. PLoS Genet. 2011;7:e1002088. PMID: 21637789 DOI: 10.1371/journal.pgen.1002088 Medline
Siddiqui QUA, Anjum S, Zahra F, Yousuf SM. Ovarian reserve parameters and response to controlled ovarian stimulation in infertile patients. Pak J Med Sci. 2019;35:958-62. PMID: 31372124 DOI: 10.12669/pjms.35.4.753 Medline
Stolk L, Perry JR, Chasman DI, He C, Mangino M, Sulem P, Barbalic M, Broer L, Byrne EM, Ernst F, Esko T, Franceschini N, Gudbjartsson DF, Hottenga JJ, Kraft P, McArdle PF, Porcu E, Shin SY, Smith AV, van Wingerden S, et al. Meta-analyses identify 13 loci associated with age at menopause and highlight DNA repair and immune pathways. Nat Genet. 2012;44:260-8. PMID: 22267201 DOI: 10.1038/ng.1051 Medline
Sullivan SD, Welt C, Sherman S. FMR1 and the continuum of primary ovarian insufficiency. Semin Reprod Med. 2011;29:299-307. PMID: 21969264 DOI: 10.1055/s-0031-1280915 Medline
Tal R, Seifer DB. Ovarian reserve testing: a user’s guide. Am J Obstet Gynecol. 2017;217:129-40. PMID: 28235465 DOI: 10.1016/j.ajog.2017.02.027 Medline
Trevisan CM, de Oliveira R, Christofolini DM, Barbosa CP, Bianco B. Effects of a Polymorphism in the Promoter Region of the Follicle-Stimulating Hormone Subunit Beta (FSHB) Gene on Female Reproductive Outcomes. Genet Test Mol Biomarkers. 2019;23:39-44. PMID: 30585745 DOI: 10.1089/gtmb.2018.0182 Medline
Voltmer-Irsch S, Kneissel S, Adenot PG, Schmidt-Zachmann MS. Regulatory mechanisms governing the oocyte-specific synthesis of the karyoskeletal protein NO145. Cell Sci. 2007;120:1412-22. PMID: 17374641 DOI: 10.1242/jcs.000166 Medline
Yang F, Wang PJ. The mammalian synaptonemal complex: a scaffold and beyond. Genome Dyn. 2009;5:69-80. PMID: 18948708 DOI: 10.1159/000166620 Medline
Zhou J, Stein P, Leu NA, Chmátal L, Xue J, Ma J, Huang X, Lampson MA, Schultz RM, Wang PJ. Accelerated reproductive aging in females lacking a novel centromere protein SYCP2L. Hum Mol Genet. 2015;24:6505-14. PMID: 26362258 DOI: 10.1093/hmg/ddv359 Medline