JBRA Assist. Reprod. 2020;00(0):00-00
ORIGINAL ARTICLE
doi: 10.5935/1518-0557.20200042
Effectiveness of the use of Low Molecular Heparin in patients with repetition abortion history: Systematic review and meta-analysis
1State University of Bahia (UNEB) - Life Sciences Department. Salvador - Bahia - Brazil
CONFLICT OF INTERESTThe authors declare no conflict of interest.
ABSTRACT
Objective: To evaluate the efficacy and safety of using low molecular weight heparin
(LMWH) in women with a history of recurrent abortion without an identified
cause.
Methods: To develop a systematic review to select the studies. Total found 437 papers.
Seven studies were completed or requested. The following variables were
analyzed: clinical pregnancy, implantation rate, live births, abortion,
premature birth, pregnancy, continuous pregnancy, beyond the 20th gestational week, congenital abnormality, hemorrhage, preeclampsia,
placental premature detachment.
Results: The LMWH group had a higher incidence of continuous pregnancy after the
20th week of gestation and there was no significant
difference between the LMWH group and the expectant management group in the
analysis of other variables.
Conclusions: There was no data showing risk and/or less use of LMWH compared to expectant
management; on the contrary, LMWH use increased the incidence of
evolutionary pregnancy after the 20th gestational week. LMWH has
some influence on prophylactic treatment of repeat abortion for unknown
cause.
Keywords: Recurrent abortion, Low molecular weight heparin, Expectant conduct, Live births
INTRODUCTION
Repeat abortion, also called habitual gestational loss, was defined, according to Stirrat (1990), as the successive
spontaneous cessation of three or more pregnancies with 20-22 gestational weeks or
less (Montenegro & Rezende Filho, 2008).
However, according to the Practice Committee of the American Society for
Reproductive Medicine (2008), habitual abortion is considered when there is two or
more consecutive gestational losses.
Habitual gestational loss is a common obstetric complication that affects about 5% of
pregnant women (Practice Medicine, 2012; Rai &
Regan, 2006). There are Several factors that may be involved in the
etiology of recurrent miscarriages, including genetic, anatomical (arcuate,
bicornuate, septate, T-shaped and unicornuate uterus), endocrine abnormalities
(luteal phase insufficiency), hyperprolactinemia, thyroid disease, polycystic ovary
syndrome, immunological, infectious, metabolic, thrombophilia and maternal age
(Burlá et al., 2015).
However, around 50% of cases have no identified cause (Practice Committee of the
American Society for Reproductive Medicine, 2012).
Considering that episodes of thrombosis in the uteroplacental circulation occur
independently of the presence of thrombophilia (Badawy et al., 2008; Nelson & Greer, 2008), some authors began to institute empirical
prophylactic treatment with low molecular weight heparin (LMWH) and/or small doses
of aspirin in women with habitual miscarriage of unexplained cause (Badawy et al., 2008; Pasquier et al., 2015). Although there are already studies
indicating the benefit of thromboprophylaxis (Badawy et al., 2008; Fawzy et al., 2008; Shaaban et al., 2017; Urman et al., 2009), there are
also studies showing that there are no significant differences in the outcome of
pregnancies of women undergoing LMWH. Therefore, this strategy should not be
instituted as routine practice until there are studies that prove the exact
mechanism of action and the benefit of this therapy in repetitive abortions
(Pasquier et al., 2015; Schleussner et al., 2015; Di
Nisio et al., 2005; Kaandorp et al., 2010; Clark et al., 2010; de
Jong et al., 2014; Visser et al., 2011).
Failure to implant in couples undergoing assisted reproduction is also a relatively
common phenomenon that affects their feelings of frustration and despair.
Implantation failure, as well as habitual abortion, has been attributed to a number
of factors; however, most have no particular cause. Knowledge about the contribution
of coagulation disorders in the implantation failure process underlies the use of
anticoagulants during assisted reproduction therapy (Urman et al., 2009). The success of this therapy can be
assessed by the implantation rate, which is defined as the number of observed
gestational sacs divided by the number of embryos transferred (Zegers-Hochschild et al., 2009).
Thus, considering the existence of still inconclusive studies and, knowing the
emotional repercussions of spontaneous abortion that involves feelings of loss and
blame for the impossibility of completing the pregnancy, this requires adequate,
safe and humanized technical attention. This study aims to evaluate the efficacy and
safety of LMWH use in women with a history of recurrent miscarriage without an
identified cause.
MATERIAL AND METHODS
Evidence Acquisition
To describe the results of this meta-analysis, we used the Preferred Reporting
Items for Systematic Reviews and Meta-Analyzes (PRISMA) (Moher et al., 2009). This systematic
review is registered in the PROSPERO database under registration number:
CRD42017082373.
Study goal
To establish the focus of the systematic review, we used the following clinical
issues: the population studied, the intervention and comparisons, study design
and study results from which we extracted the data. Thus, the goal of this
systematic review was “to detect the efficacy of LMWH in the prophylactic
treatment of women with recurrent abortion compared with the expectant
management”. We used only randomized and quasi-randomized studies, and we
structured the meta-analysis following the “PICOS” format (Population,
Intervention, Comparison, Results, Type of study) (Howick, 2009) (Table
1).
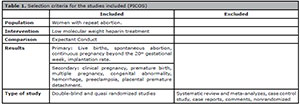
Table 1. Selection criteria for the studies included (PICOS)
• P: Women with repeat abortion history.
• I: Low molecular weight heparin treatment
• C: Expectant conduct
• O: Effectiveness
• S: Randomized and quasi-randomized studies
Eligibility criteria
The selected studies had to include women with recurrent abortion with two or
more consecutive gestational losses; to compare the efficacy of LMWH versus the expectant management in prophylactic treatment
of habitual abortion, and to be a randomized or quasi-randomized trial.
The studies were not included if they were published in summary format, letter to
the editor, comments, meta-analysis, review article, or studies that included
any drug other than LMWH in the study population that included women with repeat
abortion of known cause.
Study strategy (Appendix 1)
We carried out an electronic search in MEDLINE and PubMed in October 2017. There
was no language restriction for the papers. We based the study on the following
combined Medical Subject Headings of the National Library of Medicine (MeSH)
terms: "habitual abortion", "anticoagulants", "watchful waiting", "gestational
age", "fetal death", "live births", "premature birth", "prolonged pregnancies",
"perinatal death", "intrauterine growth retardation", "congenital abnormality",
"obstetric complications", "pregnancy associated hypertension", "pre-eclampsia",
"placenta previa", "placental abruption", "uterine hemorrhage", "postpartum
hemorrhage".
Study selection
Two researchers (ATBD and SAO) selected the publications independently.
Initially, we assessed the titles and abstracts of all the studies found by the
research strategy. Any divergence in study selection and/or data extraction was
cleared by consensus between the two researchers. We read the papers that had
insufficient information in the title and abstract in their entirety. Only
studies that had the inclusion criteria and did not meet the non-inclusion
criteria were selected for the meta-analysis. We generated a list of potential
studies for inclusion in the systematic review. We checked references from
reviews and meta-analyzes to find papers that could possibly meet the inclusion
criteria.
Data collection process
Two researchers (ATBD and SAO) independently extracted data using a standardized
form and, again, disagreements were solved by consensus. We extracted and
combined the data from all included items reporting intervention and patient
outcomes. These authors evaluated the eligibility and quality of the studies
and, subsequently, extracted data from the papers. The standardized form
included information such as authors, journal, year of publication, design,
duration and place of study, demographics of participants, inclusion and
exclusion criteria, type of interventions, and outcomes.
Data and results
We combined the studies in groups, according to the interventions performed and
the outcomes found. We combined the data, to run the following analyzes:
• Is LMWH efficient for prophylactic treatment of recurrent abortion?
• What is the best conduct for prophylactic treatment of habitual abortion?
• Which approach causes fewer adverse effects as congenital abnormality, bleeding, preeclampsia and placental premature detachment?
• Which conduct best prolongs pregnancy beyond 20 gestational weeks?
• What is the best conduct to increase implantation rate?
• Which conduct generates the most premature births?
• Which conduct contributes to the higher number of multiple pregnancies?
Partiality assessment risk
We followed the guidelines suggested by the Cochrane Collaboration group to
assess the risk of partiality studies (Higgins
& Green, 2011). Sequence generation, allocation concealment,
blinding, and incomplete outcome data were evaluated for each trial included in
the review. A low risk of bias was considered when a "yes" judgment for all
domains was obtained, while a high risk of bias was considered when a "no"
judgment for one or more domains was obtained. Table 2 depicts the quality assessment of the included trials.
Analysis
To accomplish the meta-analysis, we used the Cochrane Collaboration's Review
Manager Software (RevMan 5.3; <http://tech.cochrane.org/revman>). The
metanalytic measure of interest is the odds ratio, which we obtained using the
Mantel-Haenszel method. In cases where the number of events in one of the groups
was zero, we used the Peto's method. In addition to the odds ratios, the
respective 95% confidence intervals (CI) as well as the forest plot were
presented. We assessed the heterogeneity between the studies by the Higgins and
Thompson I2 statistics and the Cochran Q test. We applied the random
effect model when the I2 statistic was higher than 50%, or when the
null hypothesis of the Cochran Q test was rejected. The statistical tests
applied were bilateral and the adopted significance level was 5%.
We conducted a systematic literature search to identify randomized and
quasi-randomized trials comparing LMWH use and expectant conduct in the
prophylactic treatment of repeat abortion. In total, we found 437 papers as we
can see in Figure 1. At the end of the
review process, 7 papers met the inclusion criteria and were described and
evaluated (Badawy et al.,
2008; Pasquier et
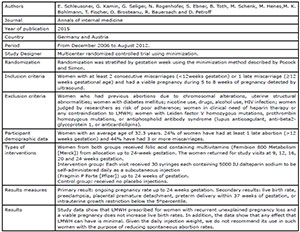
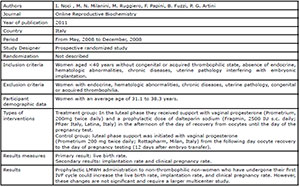
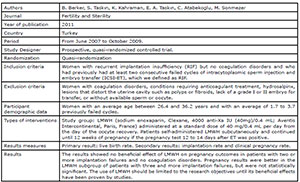
al., 2015; Shaaban et al., 2017; Urman et al., 2009; Schleussner et al., 2015; Berker et al., 2011; Noci et al., 2011) (Fig. 1). There were six randomized studies and one
quasi-randomized study. We excluded 433 studies, because they either did not
meet the inclusion criteria or did not provide sufficient data for inclusion in
the meta-analysis, or were literature review studies. From these studies, 280
evaluated women with a determined cause of miscarriage as thrombophilia, 80
included aspirin with or without LMWH in the intervention group or had women on
aspirin as a control group. We added three studies identified in the
meta-analyses’ references that met the inclusion criteria. We also included
studies evaluating the efficacy of LMWH in the prophylactic treatment of repeat
abortion in pregnant women by in vitro fertilization. Table 2 shows the quality assessment of the included
studies.
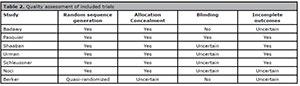
Table 2. Quality assessment of included trials
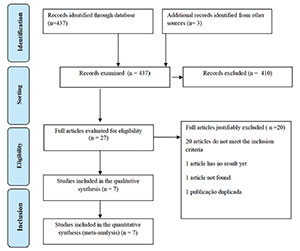
Figure 1. Study Selection Flowchart
RESULTS
Description of included studies (Appendix 2)
The included studies represented 1,855 patients (936 undergoing LMWH and 919 in
the expectant management group). Appendix 1 shows the summary characteristics of
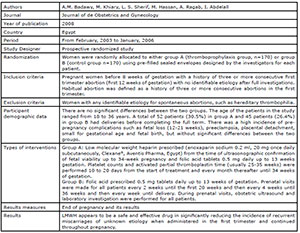
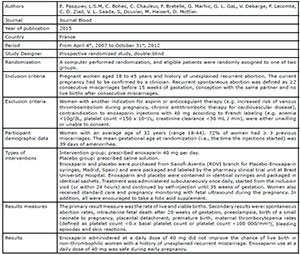
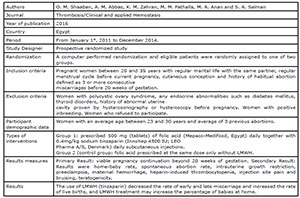
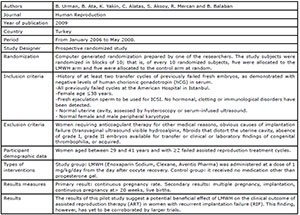
the studies included in this review. Four studies (Badawy et al., 2008; Pasquier et al., 2015; Shaaban et al., 2017; Schleussner et al.,
2015) evaluated the use of LMWH prevention of indeterminate recurrent
miscarriage and the other three studies (Urman et al., 2009; Berker et al., 2011; Noci et al., 2011) evaluated the effect of
LMWH on implantation rates in women with recurrent implantation failure (RIF),
but without coagulation disorders.
Clinical pregnancy
We assessed the number of women who reached clinical pregnancy after treatment.
We analyzed this in only three articles (Berker et al., 2011; Noci et al., 2011; Urman et al., 2009). When comparing LMWH versus expectant management, there was no statistically
significant difference between the groups (RR=1.20; 95% CI: 0.83, 1.75;
I2=0%; p=0.33) (Fig. 2).
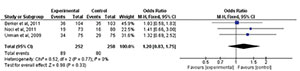
Figure 2. Forest-plot of clinical pregnancy incidence with treatment
Implantation rate
Three studies (Berker et al.,
2011; Noci et al.,
2011; Urman et al.,
2009) evaluated the implantation rate after treatment. When comparing
the two interventions there was no statistically significant difference
(RR=1.21; 95% CI: 0.88, 1.65; I2=0%; p=0.24) (Fig. 3).
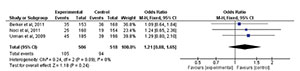
Figure 3. Forest-plot of implantation rate incidence with treatment
Live births
There were five studies (Berker et
al., 2011; Noci et al., 2011; Pasquier et al., 2015; Schleussner et al., 2015; Urman et al., 2009) that
evaluated the incidence of live births after treatment. When comparing the five
studies, we found no significant static difference between the groups (RR=1.02;
95% CI: 0.77, 1.34; I2=0%; p=0.91) (Fig. 4).
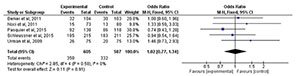
Figure 4. Forest-plot of live birth incidence with treatment
Spontaneous abortion
We assessed the presence of spontaneous abortion with the institution of
treatment. Four papers evaluated this outcome (Badawy et al., 2008; Noci et al., 2011; Pasquier et al., 2015; Shaaban et al., 2017).
When comparing the two interventions, there was no statistically significant
difference between the groups (RR=0.69; 95% CI: 0.31, 1.50; I2=83%; p=0.35) (Fig. 5).

Figure 5. Forest-plot of spontaneous abortion incidence with treatment
Premature birth
We evaluated the number of premature births that occurred after treatment. It was
possible to analyze it in only two studies (Badawy et al., 2008; Urman et al., 2009). When comparing LMWH versus expectant management, there was no statistically
significant difference between the groups (RR=0.96; 95% CI: 0.56, 1.66;
I2=5%; p=0.89) (Fig. 6).

Figure 6. Forest-plot of premature birth incidence with treatment
Multiple pregnancy
We could analyze only two papers (Berker et al., 2011; Urman et al., 2009). When comparing the two
treatments, there was no statistically significant difference between the groups
(RR=1.02; 95% CI: 0.63, 1.63; I2=0%; p=0.94) (Fig. 7).

Figure 7. Forest-plot of multiple pregnancies incidence with treatment
Continuous pregnancy beyond the 20th gestational week
Three studies (Schleussner et
al., 2015; Shaaban et al., 2017; Urman et al., 2009) evaluated the incidence of
continuous pregnancy beyond the 20th gestational week with the
institution of treatment. When comparing the two treatments, there was a
statistically significant difference between the groups (RR=2.55; 95% CI: 1.79,
3.63; I2=4%; p=0.00001). Treatment with low
molecular weight heparin resulted in a higher incidence of continuous pregnancy
beyond the 20th gestational week (Fig.
8).

Figure 8. Forest-plot of continuous pregnancy beyond the 20th gestational week incidence with treatment
Congenital abnormality
Two studies (Badawy et al.,
2008; Pasquier et
al., 2015) evaluated the incidence of congenital abnormality
after treatment. When comparing the two interventions, there was no
statistically significant difference (RR=2.46; 95% CI: 0.78, 7.79;
I2=0%; p=0.13) (Fig.
9).

Figure 9. Forest-plot of congenital abnormality incidence with treatment
Hemorrhage
Two studies (Badawy et al.,
2008; Pasquier et
al., 2015) evaluated the incidence of hemorrhage after
treatment. When comparing the two studies, there was no statistically
significant difference between the groups (RR=1.54; 95% CI: 0.92, 2.57;
I2=0%; p=0.10) (Fig. 10).

Figure 10. Forest-plot of hemorrhage incidence with treatment
Preeclampsia
We could analyze only two studies regarding the incidence of preeclampsia (Badawy et al., 2008; Shaaban et al., 2017).
When comparing LMWH versus expectant management there was no
statistically significant difference between the groups (RR=2.83; 95% CI: 0.13,
61.35; I2=91%; p=0.51) (Fig. 11).

Figure 11. Forest-plot of preeclampsia incidence with treatment
Placental premature detachment
We evaluated the presence of placental premature detachment as a side effect
during treatment. Only two papers evaluated this outcome (Badawy et al., 2008; Schleussner et al., 2015). When comparing
the two interventions, there was no statistically significant difference between
the groups (RR=0.55; 95% CI: 0.12, 2.60; I2=0%; p=0.45) (Fig. 12).

Figure 12. Forest-plot of placental premature detachment incidence with
treatment
DISCUSSION
In this study, we demonstrated evidence of LMWH not being inferior to the expectant
management. The LMWH group had a higher incidence of evolutionary pregnancy beyond
the 20th gestational week without including unfavorable factors during
pregnancy, since there was no increase in variants such as hemorrhage, preeclampsia,
placental premature detachment and preeclampsia in this group. However, it is
important to highlight that this outcome was evaluated in only three studies (Shaaban et al., 2017; Urman et al., 2009; Schleussner et al., 2015),
which demonstrates the need for a larger number of studies evaluating pregnancies
that continue beyond the 20th gestational week.
The rate of spontaneous abortion is a variable of great interest when assessing
prophylactic treatments for recurrent miscarriage, and in this study this variable
showed no statistically significant difference (RR=0.69; 95% CI: 0.31, 1.50 (83%; p=0.35). However, there is a tendency towards favoring LMWH,
but the four studies analyzed (Badawy et
al., 2008; Pasquier et al., 2015; Shaaban et al., 2017; Noci et al., 2011) have a high heterogeneity rate
(I2=83%), thus requiring more studies for the conclusion to become
more reliable.
The use of LMWH showed no statistically significant difference in the implantation
rate analysis, when compared to the expectant management (RR=1.21; 95% CI: 0.88,
1.65; I2=0%; p=0.24). However, only three studies (Urman et al., 2009; Berker et al., 2011; Noci et al., 2011) were
evaluated by the meta-analysis, indicating that there is also a need for further
studies to reach a more reliable conclusion about the action and safety of using
LMWH to treat implantation failures.
The preeclampsia variable showed a high heterogeneity between studies
(I2=91%). For this variable, two studies were evaluated (Badawy et al., 2008; Shaaban et al., 2017). In the
first, the control group had a higher incidence of preeclampsia compared to the
intervention group, unlike the second study in which the highest incidence of
preeclampsia occurred in the intervention group. The presence of high heterogeneity
of this variable makes it questionable the combination of the results of these
studies for this outcome.
In their meta-analysis, Di Nisio et
al. (2005) compared aspirin, unfractionated heparin and LMWH
use compared with one another, or a placebo to prevent birth loss in pregnant women
or women who were trying to become pregnant and who had a history of at least two
consecutive abortions with no apparent causes. One of the included studies (Gris et al., 2004) resulted in
increased live birth rates compared to low aspirin doses. Four patients had
preeclampsia in the enoxaparin intervention group, and three participants in the
aspirin group had it. There was one case of premature birth in the aspirin
intervention group. However, since the studies included in the meta-analysis had a
small sample size and methodological limitations, they concluded that
thromboprophylaxis should not be prescribed until convincing data exists.
There was a meta-analysis in 2009 (Kaandorp et al., 2009), evaluating the efficacy and safety of
aspirin, LMWH and fractional heparin compared with one another or with placebo in
women with a history of at least two miscarriages of spontaneous causes. The rate of
live births was similar between the enoxaparin group (82%) and the aspirin group
(84%) (RR 0.97; 95% CI: 0.81 to 1.16). Three women had preeclampsia in the aspirin
group and no women had preeclampsia in the enoxaparin group. In each group, there
was one birth with congenital abnormalities. None of the studies showed greater
efficacy of one treatment over the other, so the meta-analysis concluded that the
use of anticoagulants should not be recommended.
In 2014, nine studies included in a meta-analysis (de
Jong et al., 2014) reviewed the effects of LMWH,
fractional heparin or aspirin, or a combination of both compared with one another or
to placebo in the prophylactic treatment of pregnant women with a history of at
least two miscarriages. There were three studies assessing the LMWH effects (Badawy et al., 2008; Fawzy et al., 2008; Martinelli et al., 2012).
There were no differences between treatment groups among individual studies for
gestational complications, bleeding or thromboembolic events. Based on these
results, the review showed that the use of anticoagulants in women with recurrent
miscarriage was not effective.
Although these meta-analyses do not specifically compare LMWH and expectant
management, they are important because they show in their results that patients who
received prophylactic LMWH treatment had statistically insignificant results when
compared to other treatments, which differs from our study, that already presents
statistically significant differences favoring the LMWH group when analyzing the
continuous pregnancy beyond the 20th gestational week variable.
We believe this study contributes to the still unresolved debate about the use of
LMWH in the prophylactic treatment of recurrent miscarriage. When comparing this
intervention with expectant management, there was no data to show risk and/or lower
efficacy of LMWH. On the contrary, LMWH was more effective in increasing the
incidence of evolutionary pregnancy beyond the 20th gestational week;
thus indicating that LMWH has some influence on the prophylactic treatment of
recurrent miscarriage of unknown cause.
Therefore, we need further studies with standardized methods to evaluate the
comparison of LMWH and expectant management.
ACKNOWLEDGMENTS
We thank Vinícius Fernando Calsavara who helped us
with the statistical analysis
Appendix 1. Research Strategy
2 Abortions, Habitual) OR (Habitual Abortion) OR (Habitual Abortions) OR
(Miscarriage, Recurrent) OR (Miscarriages, Recurrent) OR (Recurrent Miscarriage)
OR (Recurrent Miscarriages) OR (Abortion, Recurrent) OR (Abortions, Recurrent)
OR (Recurrent Abortion) OR (Recurrent Abortions)
5 (Anticoagulation Agents) OR (Agents, Anticoagulation) OR (Anticoagulant Agents)
OR (Agents, Anticoagulant) OR (Anticoagulant Drugs) OR (Drugs, Anticoagulant) OR
(Anticoagulant) OR (Indirect Thrombin Inhibitors) OR (Inhibitors, Indirect
Thrombin) OR (Thrombin Inhibitors, Indirect)
7(Waiting, Watchful) OR (Watchful Waiting) OR (Watchful Waiting)
10 (Age, Gestational) OR (Ages, Gestational) OR (Gestational Ages) OR (Maturity,
Chronologic Fetal) OR (Chronologic Fetal Maturity) OR (Fetal Maturity,
Chronologic) OR (Fetal Age) OR (Age, Fetal) OR (Ages, Fetal) OR (Fetal Ages)
13 (Fetal Death) OR (Deaths, Fetal) OR (Fetal Deaths) OR (Fetal Demise) OR
(Demise, Fetal) OR (Fetal Mummification) OR (Mummification, Fetal)
15 (Live Births)
17 (Birth, Premature) OR (Births, Premature) OR (Premature Births) OR (Preterm
Birth) OR (Birth, Preterm) OR (Births, Preterm) OR (Preterm Births)
19 (Pregnancies, Prolonged) OR (Prolonged Pregnancies) OR (Prolonged
Pregnancy)
21 (Death, Perinatal) OR (Deaths, Perinatal) OR (Perinatal Deaths) OR (Neonatal
Death) OR (Death, Neonatal) OR (Deaths, Neonatal) OR (Neonatal Deaths)
24 (Growth Retardation, Fetal) OR (Retardation, Fetal Growth) OR (Intrauterine
Growth Retardation) OR (IUGR) OR (Growth Retardation, Intrauterine) OR
(Retardation, Intrauterine Growth)
27 (Abnormality, Congenital) OR (Congenital Abnormality) OR (Deformities) OR
(Deformity) OR (Congenital Defects) OR (Congenital Defect) OR (Defect,
Congenital) OR (Defects, Congenital) OR (Abnormalities, Congenital) OR (Birth
Defects) OR (Birth Defect) OR (Defect, Birth) OR (Defects, Birth)
29 (Complication, Obstetric Labor) OR (Complications, Obstetric Labor) OR (Labor
Complication, Obstetric) OR (Labor Complications, Obstetric) OR (Obstetric Labor
Complication) OR (Labor Complications) OR (Complication, Labor) OR (Labor
Complication) OR (Complications, Labor)
31 (Hypertension, Pregnancy Induced) OR (Pregnancy-Induced Hypertension) OR
(Pregnancy Induced Hypertension) OR (Hypertensions, Pregnancy Induced) OR
(Induced Hypertension, Pregnancy) OR (Induced Hypertensions, Pregnancy) OR
(Gestational Hypertension) OR (Hypertension, Gestational) OR (Transient
Hypertension, Pregnancy) OR (Hypertension, Pregnancy Transient) OR (Pregnancy
Transient Hypertension)
33 (Pre-Eclampsia) OR (Preeclampsia) OR (Pregnancy Toxemias) OR (Pregnancy
Toxemia) OR (Toxemia, Pregnancy) OR (Edema-Proteinuria-Hypertension Gestosis) OR
(Edema Proteinuria Hypertension Gestosis) OR (Gestosis,
Edema-Proteinuria-Hypertension) OR (Hypertension-Edema-Proteinuria Gestosis) OR
(Gestosis, Hypertension-Edema-Proteinuria) OR (Hypertension Edema Proteinuria
Gestosis) OR (Toxemia Of Pregnancy) OR (Of Pregnancies, Toxemia) OR (Of
Pregnancy, Toxemia) OR (Pregnancies, Toxemia Of) OR (Pregnancy, Toxemia Of) OR
(Toxemia Of Pregnancies) OR (EPH Complex) OR (EPH Toxemias) OR (EPH Toxemia) OR
(Toxemia, EPH) OR (Toxemias, EPH) OR (EPH Gestosis) OR (Gestosis, EPH) OR
(Toxemias, Pregnancy) OR (Preeclampsia Eclampsia 1) OR (1, Preeclampsia
Eclampsia) OR (1s, Preeclampsia Eclampsia) OR (Eclampsia 1, Preeclampsia) OR
(Eclampsia 1s, Preeclampsia) OR (Preeclampsia Eclampsia 1s) OR
(Proteinuria-Edema-Hypertension Gestosis) OR (Gestosis,
Proteinuria-Edema-Hypertension) OR (Proteinuria
Edema Hypertension Gestosis)
36 (Placental Abruption) OR (Abruption, Placental) OR (Abruptions, Placental) OR
(Placental Abruptions)
38 (Uterine Hemorrhages) OR (Hemorrhage, Uterine) OR (Uterine Bleeding) OR
(Bleeding, Uterine) OR (Uterine Bleedings) OR (Vaginal Bleeding) OR (Bleeding,
Vaginal) OR (Bleedings, Vaginal) OR (Vaginal Bleedings)
40 (Hemorrhage, Postpartum) OR (Immediate Postpartum Hemorrhage) OR (Hemorrhage,
Immediate Postpartum) OR (Postpartum Hemorrhage, Immediate) OR (Delayed
Postpartum Hemorrhage) OR (Hemorrhage, Delayed Postpartum) OR (Postpartum
Hemorrhage, Delayed)
41(2 AND 5) AND 10
42(2 AND 5) AND 13
43(2 AND 5) AND 15
44(2 AND 5) AND 17
45(2 AND 5) AND 19
46(2 AND 5) AND 21
47(2 AND 5) AND 24
48(2 AND 5) AND 27
49(2 AND 5) AND 29
50(2 AND 5) AND 31
51(2 AND 5) AND 33
52(2 AND 5) AND 36
53(2 AND 5) AND 38
54(2 AND 5) AND 40
55(2 AND 7) AND 10
56(2 AND 7) AND 13
58(2 AND 7) AND 15
59(2 AND 7) AND 15 Schema: all
60(2 AND 7) AND 17
61(2 AND 7) AND 19
62(2 AND 7) AND 21
63(2 AND 7) AND 21 Schema: all
64(2 AND 7) AND 24
65(2 AND 7) AND 24 Schema: all
66(2 AND 7) AND 27
67(2 AND 7) AND 27 Schema: all
68(2 AND 7) AND 29
69(2 AND 7) AND 31
70(2 AND 7) AND 31 Schema: all
71(2 AND 7) AND 33
72(2 AND 7) AND 33 Schema: all
73(2 AND 7) AND 36
74(2 AND 7) AND 36 Schema: all
75(2 AND 7) AND 38
76(2 AND 7) AND 38 Schema: all
77(2 AND 7) AND 40
78(2 AND 7) AND 40 Schema: all
79((2 AND 5) AND 10)) OR ((2) AND 5) AND 13)) OR ((2) AND 5) AND 15)) OR ((2) AND
5)
AND 17)) OR ((2) AND 5) AND 19)) OR ((2) AND 5) AND 21)) OR ((2) AND 5) AND 24))
OR ((2)
AND 5) AND 27)) OR ((2) AND 5) AND 29)) OR ((2) AND 5) AND 31)) OR ((2) AND 5)
AND 33))
OR ((2) AND 5) AND 36)) OR ((2) AND 5) AND 38)) OR ((2) AND 5) AND 40)) OR ((2)
AND 7)
AND 10)) OR ((2) AND 7) AND 15)) OR ((2) AND 7) AND 17)) OR ((2) AND 7) AND 19))
OR ((2)
AND 7) AND 21)) OR ((2) AND 7) AND 24)) OR ((2) AND 7) AND 27)) OR ((2) AND 7)
AND 29))
OR ((2) AND 7) AND 31)) OR ((2) AND 7) AND 33)) OR ((2) AND 7) AND 36)) OR ((2)
AND 7)
AND 38)) OR ((2) AND 7) AND 40)) OR ((2) AND 7) AND 13)
Appendix 2. Characteristics of included studies
Badawy et al., 2008
Pasquier et al., 2015
Shaaban et al., 2017
Urman et al., 2009
Schleussner et al., 2015
Noci et al., 2011
Berker et al., 2011
REFERENCES
Badawy AM, Khiary M, Sherif LS, Hassan M, Ragab A, Abdelall I.
Low-molecular weight heparin in patients with recurrent early miscarriages of
unknown aetiology. J Obstet Gynaecol. 2008;28:280-4. PMID: 18569468 DOI:
10.1080/01443610802042688
Medline Crossref
Berker B, Taşkin S, Kahraman K, Taşkin EA,
Atabekoğlu C, Sönmezer M. The role of low-molecular-weight heparin in
recurrent implantation failure: a prospective, quasi-randomized, controlled
study. Fertil Steril. 2011;95:2499-502. PMID: 21244861 DOI:
10.1016/j.fertnstert.2010.12.033
Medline Crossref
Clark P, Walker ID, Langhorne P, Crichton L, Thomson A, Greaves M,
Whyte S, Greer IA; Scottish Pregnancy Intervention Study (SPIN) collaborators.
SPIN (Scottish Pregnancy Intervention) study: a multicenter, randomized
controlled trial of low-molecular-weight heparin and low-dose aspirin in women
with recurrent miscarriage. Blood. 2010;115:4162-7. PMID: 20237316 DOI:
10.1182/blood-2010-01-267252
Medline Crossref
de Jong PG, Kaandorp S, Di Nisio M, Goddijn M, Middeldorp S. Aspirin
and/or heparin for women with unexplained recurrent miscarriage with or without
inherited thrombophilia. Cochrane Database Syst Rev. 2014;7:CD004734. PMID:
24995856 DOI: 10.1002/14651858.CD004734.pub4
Medline Crossref
Di Nisio M, Peters L, Middeldorp S. Aspirin or anticoagulants for
the treatment of recurrent miscarriage in women without antiphospholipid
syndrome. Cochrane Database Syst Rev. 2005;2:CD004734. PMID: 15846729 DOI:
10.1002/14651858.CD004734.pub2
Medline Crossref
Fawzy M, Shokeir T, El-Tatongy M, Warda O, El-Refaiey AA, Mosbah A.
Treatment options and pregnancy outcome in women with idiopathic recurrent
miscarriage: a randomized placebo-controlled study. Arch Gynecol Obstet.
2008;278:33-8. PMID: 18071727 DOI: 10.1007/s00404-007-0527-x
Medline Crossref
Gris JC, Mercier E, Quéré I, Lavigne-Lissalde G, Cochery-Nouvellon
E, Hoffet M, Ripart-Neveu S, Tailland ML, Dauzat M, Marès P.
Low-molecular-weight heparin versus low-dose aspirin in women with one fetal
loss and a constitutional thrombophilic disorder. Blood. 2004;103:3695-9. PMID:
14739212 DOI: 10.1182/blood-2003-12-4250
Medline Crossref
Kaandorp S, Di Nisio M, Goddijn M, Middeldorp S. Aspirin or
anticoagulants for treating recurrent miscarriage in women without
antiphospholipid syndrome. Cochrane Database Syst Rev. 2009;1:CD004734. PMID:
19160241 DOI: 10.1002/14651858.CD004734.pub3
Medline Crossref
Kaandorp SP, Goddijn M, van der Post JA, Hutten BA, Verhoeve HR,
Hamulyák K, Mol BW, Folkeringa N, Nahuis M, Papatsonis DN, Büller HR, van der
Veen F, Middeldorp S. Aspirin plus heparin or aspirin alone in women with
recurrent miscarriage. N Engl J Med. 2010;362:1586-96. PMID: 20335572 DOI:
10.1056/NEJMoa1000641
Medline Crossref
Martinelli I, Ruggenenti P, Cetin I, Pardi G, Perna A, Vergani P,
Acaia B, Facchinetti F, La Sala GB, Bozzo M, Rampello S, Marozio L, Diadei O,
Gherardi G, Carminati S, Remuzzi G, Mannucci PM; HAPPY Study Group. Heparin in
pregnant women with previous placenta-mediated pregnancy complications: a
prospective, randomized, multicenter, controlled clinical trial. Blood.
2012;119:3269-75. PMID: 22289887 DOI:
10.1182/blood-2011-11-391383
Medline Crossref
Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred
reporting items for systematic reviews and meta-analyses: the PRISMA statement.
BMJ. 2009;339:b2535. PMID: 19622551 DOI: 10.1136/bmj.b2535
Medline Crossref
Nelson SM, Greer IA. The potential role of heparin in assisted
conception. Hum Reprod Update. 2008;14:623-45. PMID: 18701511 DOI:
10.1093/humupd/dmn031
Medline Crossref
Noci I, Milanini MN, Ruggiero M, Papini F, Fuzzi B, Artini PG.
Effect of dalteparin sodium administration on IVF outcome in non-thrombophilic
young women: a pilot study. Reprod Biomed Online. 2011;22:615-20. PMID: 21498125
DOI: 10.1016/j.rbmo.2011.03.016
Medline Crossref
Pasquier E, de Saint Martin L, Bohec C, Chauleur C, Bretelle F,
Marhic G, Le Gal G, Debarge V, Lecomte F, Denoual-Ziad C, Lejeune-Saada V,
Douvier S, Heisert M, Mottier D. Enoxaparin for prevention of unexplained
recurrent miscarriage: a multicenter randomized double-blind placebo-controlled
trial. Blood. 2015;125:2200-5. PMID: 25636341 DOI:
10.1016/j.fertnstert.2008.08.065
Medline Crossref
Practice Committee of the American Society for Reproductive
Medicine. Definitions of infertility and recurrent pregnancy loss. Fertil
Steril. 2008;90:S60. PMID: 19007647 DOI:
10.1016/j.fertnstert.2008.08.065
Medline Crossref
Practice Committee of the American Society for Reproductive
Medicine. Evaluation and treatment of recurrent pregnancy loss: a committee
opinion. Fertil Steril. 2012;98:1103-11. PMID: 22835448 DOI:
10.1016/j.fertnstert.2012.06.048
Medline Crossref
Rai R, Regan L. Recurrent miscarriage. Lancet. 2006;368:601-11.
PMID: 16905025 DOI: 10.1016/S0140-6736(06)69204-0
Medline Crossref
Schleussner E, Kamin G, Seliger G, Rogenhofer N, Ebner S, Toth B,
Schenk M, Henes M, Bohlmann MK, Fischer T, Brosteanu O, Bauersachs R, Petroff D;
ETHIG II group. Low-molecular-weight heparin for women with unexplained
recurrent pregnancy loss: a multicenter trial with a minimization randomization
scheme. Ann Intern Med. 2015;162:601-9. PMID: 25938990 DOI:
10.7326/M14-2062
Medline Crossref
Shaaban OM, Abbas AM, Zahran KM, Fathalla MM, Anan MA, Salman SA.
Low-Molecular-Weight Heparin for the Treatment of Unexplained Recurrent
Miscarriage With Negative Antiphospholipid Antibodies: A Randomized Controlled
Trial. Clin Appl Thromb. 2017;23:567-72. PMID: 27572887 DOI:
10.1177/1076029616665167
Medline Crossref
Stirrat GM. Recurrent miscarriage I: definition and epidemiology.
Lancet. 1990;336:673-5. PMID: 1975862 DOI:
10.1016/0140-6736(90)92159-F
Medline Crossref
Urman B, Ata B, Yakin K, Alatas C, Aksoy S, Mercan R, Balaban B.
Luteal phase empirical low molecular weight heparin administration in patients
with failed ICSI embryo transfer cycles: a randomized open-labeled pilot trial.
Hum Reprod. 2009;24:1640-7. PMID: 19357135 DOI:
10.1093/humrep/dep086
Medline Crossref
Visser J, Ulander VM, Helmerhorst FM, Lampinen K, Morin-Papunen L,
Bloemenkamp KW, Kaaja RJ. Thromboprophylaxis for recurrent miscarriage in women
with or without thrombophilia. HABENOX: a randomized multicenter trial. Thromb
Haemost. 2011;105:295-301. PMID: 21103659 DOI:
10.1160/TH10-05-0334
Medline Crossref
Zegers-Hochschild F, Adamson GD, de Mouzon J, Ishihara O, Mansour R,
Nygren K, Sullivan E, Vanderpoel S; International Committee for Monitoring
Assisted Reproductive Technology; World Health Organization. International
Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World
Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil
Steril. 2009;92:1520-4. PMID: 19828144 DOI:
10.1016/j.fertnstert.2009.09.009
Medline Crossref