JBRA Assist. Reprod. 2024;28(3):489-496
REVIEW
doi: 10.5935/1518-0557.20230074
Fertility preservation counseling for women of reproductive age diagnosed with cancer: an integrative review
1University of São Paulo at Ribeirão Preto College of Nursing, Department Public Health Nursing, Ribeirão Preto - São Paulo, Southeast, Brazil
CONFLICT OF INTERESTS
The authors have no conflicts of interest to report.
ABSTRACT
This integrative review synthesizes the scientific evidence on fertility preservation counseling prior to oncological treatment for women of reproductive age diagnosed with cancer. Bibliographic research was conducted on databases PubMed, CINAHL, LILACS, EMBASE, Scopus, and Web of Science. The structured search strategy for the review question was “counseling AND antineoplastic agents AND fertility preservation”. The use of controlled descriptors and keywords was adapted for each database. Study selection through the Rayyan platform was independent and blinded. The final sample comprised seven studies emphasizing the importance of clarifying factors related to the risk of infertility due to oncological treatment and fertility preservation techniques, such as success rate, pregnancy rate, cost, available options, and side-effects, as well as discussing the possibilities of adoption and surrogacy. This review provided evidence reinforcing the importance of counseling for fertility preservation, promoting motherhood for women who face oncological treatment. Organized networks linking oncology and reproductive medicine units are crucial to facilitate patient referral between these services and interprofessional communication.
Keywords: directive counseling, fertility preservation, women’s health, antineoplastic agents, nursing, assisted reproductive techniques
INTRODUCTION
Cancer is estimated to have been diagnosed in 19.3 million people worldwide in 2020 (International Agency for Research on Cancer [IARC], 2020). In Brazil, 704 thousand new cases of cancer are expected to occur every year from 2023 to 2025 (Santos et al., 2023). Women of reproductive age account for 3% to 10% of all cancer diagnoses worldwide (Massarotti et al., 2021).
Women of reproductive age diagnosed with cancer require comprehensive fertility and pregnancy care due to treatment being possibly gonadotoxic. After chemotherapy and/or radiotherapy, women present a higher risk of ovarian insufficiency and early menopause, in addition to fibrosis, atrophy, and vascular lesion in reproductive organs (Taylan & Oktay, 2019).
In pelvic radiotherapy, the total dose estimated to increase the risk of early ovarian failure and severe uterine damage is 20 Gy (Tomás et al., 2016; Wo & Viswanathan, 2009). In turn, chemotherapy schemes with alkylating agents, such as cyclophosphamide, pose a higher risk of ovarian toxicity and infertility (Zhao et al., 2014).
Ovarian changes due to oncological treatment may negatively impact the reproductive plans of women or couples. The continuous discussion of fertility issues throughout survival stages, also before and after cancer therapy, is particularly important for patients who might face changes in their lives, relationship status, or attitude towards building a family (Shen et al., 2019).
Oncofertility associates reproductive endocrinology with oncology to increase the access of cancer patients to fertility preservation techniques after oncological diagnosis with the objective of improving the quality of life of cancer survivors (Rashedi et al., 2020). Health professionals must inform patients of possible threats to fertility as soon as possible in the process of diagnosis and treatment to provide a broader range of fertility preservation options (ESHRE, 2020; Oktay et al., 2018).
Fertility preservation offers women the possibility of having children after oncological treatment; their choice must be considered during counseling by professionals specializing in fertility (ESHRE, 2020; Oktay et al., 2018).
Despite the publication of directives emphasizing the importance of fertility preservation counseling as a strategy for fighting the risks of infertility related to oncological treatments, the scientific production on this theme is general, particularly regarding orientation from health professionals.
Identifying the directives for fertility preservation counseling is relevant to improve oncofertility care, given that women of reproductive age diagnosed with cancer do not feel supported in their decision-making (Del Valle et al., 2022). Progress in this type of counseling is required for these women to decide their reproductive future based on reliable information. Thus, this study had the objective of analyzing and synthesizing the scientific evidence on fertility preservation counseling prior to oncological treatment for women of reproductive age diagnosed with cancer.
MATERIAL AND METHODS
Study design
This study consists of an integrative literature review registered on the Open Science Framework (OSF) platform on 2022, available at https://osf.io/. The study had the following phases: elaboration of the review question, literature search of primary studies, primary data assessment, data analysis, and review presentation (Whittemore & Knafl, 2005).
The guiding question of this study was elaborated using the PICO (Population, Intervention, Control, and Outcome) strategy: P - Women of reproductive age diagnosed with cancer; I - Counseling before a fertility-threatening oncological treatment; C - Not applicable; O - Fertility preservation.
This strategy led to the following guiding question: “Which is the scientific evidence on fertility preservation counseling prior to a fertility-threatening oncological treatment for women of reproductive age diagnosed with cancer?”.
Search strategy
The search was conducted on September 17, 2023, on the following electronic databases: PubMed, CINAHL, LILACS, EMBASE, Scopus, and Web of Science. The controlled and uncontrolled subject descriptors were identified for structuring a specific search strategy for each database combined with the Boolean operators AND and OR (Table 1).
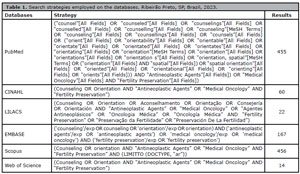
Table 1. Search strategies employed on the databases. Ribeirão Preto, SP, Brazil, 2023.
After the searches were conducted, the documents were exported to the Rayyan web app (Ouzzani et al., 2016) to help in the process of organizing and selecting articles, as well as excluding duplicates. The studies were assessed and selected by two independent, blinded reviewers in this app. In the first phase titles and abstracts were screened based on this review’s eligibility criteria. In the second phase the eligible studies were analyzed through full-text reading. Disagreements between the two reviewers were resolved in a consensus meeting. If disagreements persisted, a third reviewer with expertise on the theme was consulted.
Selection criteria
The inclusion criteria were primary articles dealing the fertility preservation counseling for women of reproductive age published in Portuguese, English, or Spanish, without date restriction. The excluded studies were those involving children, adolescents, or men and those published as editorials, protocols, in annals, as congress abstracts, and reviews.
The resulting articles amounted to 1,174 and 305 were removed due to duplication. The remaining 869 had their titles and abstracts screened, 43 of which were selected for full-text reading, whereas the other 826 were excluded. After full-text reading, 37 articles were excluded, following the eligibility criteria of this re-view. In addition, one study was identifiedin the reference list and included in the finalsample (handsearching). Thus, seven studies composed this review’s finalsample (Figure 1).
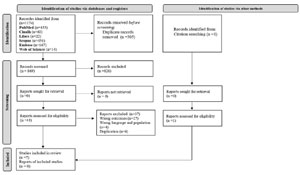
Figure 1. Flowchart of the process of selection of primary studies included in the integrative review based on Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) Source: Page et al. (2021).
Data collection instrument
The data from the selected studies were collected in a form containing the following information: authors, year of publication, country, objective, type of study, sample, main results, and conclusions. This phase was conducted by two independent reviewers and disagreements were discussed until a consensus was reached in the meetings.
Data analysis
The type of study was identified according to the classification provided by the authors of the studies included in this review. This information was not found in two of the studies, which were classified as qualitative.
The methodological quality of the included articles was assessed through instruments in accordance with the type of study proposed by the Joanna Briggs Institute’s (JBI) scientific committee. The JBI instruments are composed of questions to be answered by the reviewer with “yes”, “no”, or “unsure”. The questions assess the internal validity and risk of bias of the studies. They do not propose a score system for study assessment, but a higher number of questions replied with “yes” indicates a better methodological quality (Moola et al., 2020).
The instruments were independently applied by two reviewers and disagreements were solved in a consensus meeting between the reviewers. The data were analyzed and synthesized descriptively.
RESULTS
The flowchart for the selection process of primary stud-ies included in the integrative review (IR) is presented in Figure 1. Thus, out of the 1174 publications identified in the databases (registers), after the application of the eligibil-ity criteria, 43 primary studies were selected for full-text reading, 37 articles were excluded, one study was identifiedin the reference list and included in the final sample and 7 comprised the review sample.
Table 2 presents the descriptive synthesis of the primary studies included in this review by author, year of publication, country, language, type of study, sample, objective, and main results.
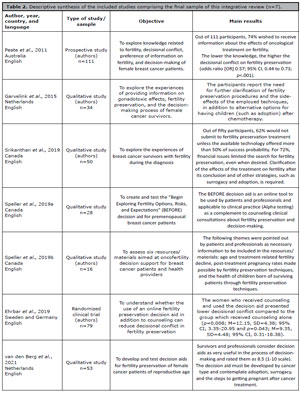
Table 2. Descriptive synthesis of the included studies comprising the final sample of this integrative review (n=7).
In the assessment of qualitative studies (n=5) with the JBI Critical Appraisal Checklist for Qualitative Research instrument, out of the 10 questions composing the checklist, four studies had 7 responses with “yes” and one study presented “yes” 8 times, as can be seen in Table 3.
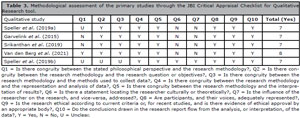
Table 3. Methodological assessment of the primary studies through the JBI Critical Appraisal Checklist for Qualitative Research tool.
In the assessment of the randomized study (n=1) with the JBI Critical Appraisal Tool for Assessment of Risk of Bias for Randomised Controlled Trials, out of the 13 questions composing the checklist, 10 had “yes” as a response, as can be observed in Table 4.

Table 4. Methodological assessment of the primary studies through the JBI Critical Appraisal Tool for Assessment of Risk of Bias for Randomised Controlled Trials.
In the assessment of the prospective study (n=1) through the JBI Critical Appraisal Tool for Assessment of Risk of Bias for Cohort Studies, out of 11 questions comprising the checklist, the study had “yes” as a response 5 times, as can be observed in Table 5.

Table 5. Methodological assessment of the primary studies through the JBI Critical Appraisal Tool for Assessment of Risk of Bias for Cohort Studies.
DISCUSSION
This study synthesized the scientific evidence related to fertility preservation counseling for women of reproductive age diagnosed with cancer, verifying that these patients wish to receive information on the fertility preservation procedures, such as cost, risks, adverse events, and success and pregnancy rates. The studies show that the possibility of becoming a mother is important for many women after a cancer diagnosis, not necessarily by giving birth, but resorting also to other strategies, such as adoption and surrogacy.
In a whole care model for women of reproductive age diagnosed with cancer, orientation on gonadotoxic effects must be provided as early as possible, before oncological therapy is started. This can be provided by the clinical care team, which must then refer the patient to a professional specializing in fertility for fertility preservation counseling (ESHRE, 2020; Kufel-Grabowska et al., 2022).
The women and the health professionals manifested different concerns related to fertility preservation. The professionals reflected on the possible information overload during diagnosis upon premature referral to fertility specialists (Speller et al., 2019a). In addition to lacking knowledge on fertility preservation, they also present questions on who must be the responsible professional for discussing this procedure, considering that this is a difficult theme and the professionals believe that it makes female patients worried (Van den Berg et al., 2021). The women wish to discuss this theme because it generates positive feelings, such as the hope of becoming mothers, and have demonstrated discomfort with having little time to make this decision before oncological therapy (Daly et al., 2019; Garvelink et al., 2015).
Such aspects compromise counseling quality and subsequent decision-making in fertility preservation. Therefore, training programs are fundamental for health professionals to enhance their knowledge of this theme and apply it in their daily work (Takeuchi et al., 2018). Training on oncofertility competences helps establishing roles and improving the implementation of guidelines and access to care (Anazodo et al., 2019).
In the face of barriers to professionals regarding oncofertility care, strategies were compiled to improve this aspect of care in clinical practice, such as the development of educational tools directed at patients (leaflets, decision aids), professional education, and the inclusion of oncological nurses with a defined role in fertility preservation (Van den Berg et al., 2019).
Decision aids are tools which provide information and clarify the agreement between decisions and personal values; they may complement counseling, as observed in three studies included in this review (Speller et al., 2019a, 2019b; Van den Berg et al., 2021). These resources, used in different contexts of the health area, help in the decision process by providing information on available options, risks, and benefits associated to each of the fertility preservation techniques, minimizing uncertainties (Gonçalves, 2021; Stacey et al., 2017). These may be printed and/or online versions, available in different countries and languages. As of this review’s literature search, no Brazilian version was found.
Decisional conflict is defined as uncertainty on whether to submit to the fertility preservation procedure. In a study assessing 111 female cancer patients of reproductive age, 63.1% presented high decisional conflict (Peate et al., 2011). Similar data was found in a study with 155 women, 62.7% of whom also presented high decisional conflict (Müller et al., 2018).
The choice for preserving fertility must be based on biopsychosocial principles, so that the health professional may help in the process of decision-making through integrated and interdisciplinary actions. Oncofertility nurse navigators may coordinate the fertility preservation counseling team, articulating care between cancer treatment centers and fertility services, in addition to the skills to identify women who will be submitted to treatment which might affect fertility and who are eligible for its preservation (Keim-Malpass et al., 2018; Semler & Thom, 2019; Zwingerman et al., 2020).
In the analyzed studies, the technique of cryopreservation was the most frequent and preservation of ovarian, egg, or embryonic tissue varied from 24 to 76.9% (Garvelink et al., 2015; Srikanthan et al., 2019; Van den Berg et al., 2021). The literature shows that cryopreservation of eggs and embryos is a consolidated technique with a favorable gestational outcome among reproductive-age women with an indication for oncological treatments considered to be gonadotoxic (ESHRE, 2020).
Counseling should discuss the possibility of surrogacy as an alternative option for motherhood, particularly for women who are temporarily or permanently unable to carry a pregnancy after oncological treatment or choose not to do so, particularly in cases of pelvic radiotherapy (ESHRE, 2020). Adoption is also an alternative for building a family after cancer treatment depending on the cultural and legal characteristics of each country (Rashedi et al., 2020).
The high financial cost of the procedures creates difficulties in choosing fertility preservation (Anazodo et al., 2019; Silva et al., 2021; Srikanthan et al., 2019). The costs vary with the laws regulating the health systems of each country, regardless of their status as developed or in development. In Canada, cryopreservation of eggs and embryos varies from being free of charge to costing thousands of Canadian dollars; despite Canada’s public and universal healthcare system, coverage of procedures differs among provinces (Brandão, 2019; Speller et al., 2019a). In Brazil, the same procedure costs twenty minimum wages and is neither provided by the Unified Health System (Sistema Único de Saúde - SUS) nor listed as a procedure by the National Supplementary Health Agency (Agência Nacional de Saúde Suplementar - ANS).
The limitations of this study are related to a lack of data regarding the role of relatives and partners in the process of fertility preservation decision-making, given that these are essential figures in the perspective of whole care.
This is added to a lack of clarity on the singularities of the professional classes in the process of fertility preservation counseling and the fact that most studies comprising this review have been conducted with survivors.
Recently diagnosed female cancer patients are emphasized to present different needs for information; however, the survivors presented an encompassing view of treatment stages and registered fundamental aspects to be discussed during counseling. This situation might not have been possible only with studies discussing recently diagnosed female patients, given that initially the worries about the severity of this diagnosis leads to a higher priority of survival, and fertility issues are not considered during diagnosis.
Other limitations include the fact that gray literature was not considered, in addition to language restrictions. Data analysis and synthesis was descriptive, which may have led to a bias in the elaboration of the results. On the other hand, the search for primary studies was conducted on relevant databases for the health and nursing fields. The rigor in the conduction of this synthesis was strengthened by the methodological quality assessment of the studies with tools elaborated by the Joanna Briggs Institute.
CONCLUSION
The results of this review emphasize the importance of creating or improving educational resources and decision aids to enhance the process of fertility preservation counseling, minimizing decisional conflict. Women wish to understand different fertility preservation techniques and other possibilities for becoming mothers, such as adoption and surrogacy.
This review generated evidence that reinforces the importance of fertility preservation counseling, providing women facing oncological treatment with the possibility of raising children. Organized networks between oncology and reproductive medicine units are crucial to facilitate the referral of patients among services and interprofessional communication.
The study pointed out a lack of data regarding the role of relatives and partners in the process of fertility preservation decision-making, given that these are essential figures in the perspective of whole care. It is suggested that further research be carried out in order to better understand this gap.
REFERENCES
Anazodo A, Laws P, Logan S, Saunders C, Travaglia J, Gerstl B, Bradford N, Cohn R, Birdsall M, Barr R, Suzuki N, Takae S, Marinho R, Xiao S, Qiong-Hua C, Mahajan N, Patil M, Gunasheela D, Smith K, Sender L, et al. How can we improve oncofertility care for patients? A systematic scoping review of current international practice and models of care. Hum Reprod Update. 2019;25:159-79. PMID: 30462263 DOI: 10.1093/humupd/dmy038 Medline
Brandão JRM. Primary health care in Canada: current reality and challenges. Cad Saude Publica. 2019;35:e00178217. PMID: 30652821 DOI: 10.1590/0102-311x00178217 Medline
Daly C, Micic S, Facey M, Speller B, Yee S, Kennedy ED, Corter AL, Baxter NN. A review of factors affecting patient fertility preservation discussions & decision-making from the perspectives of patients and providers. Eur J Cancer Care (Engl). 2019;28:e12945. PMID: 30375696 DOI: 10.1111/ecc.12945 Medline
Del Valle L, Corchón S, Palop J, Rubio JM, Celda L. The experience of female oncological patients and fertility preservation: A phenomenology study. Eur J Cancer Care (Engl). 2022;31:e13757. PMID: 36354130 DOI: 10.1111/ecc.13757 Medline
Ehrbar V, Urech C, Rochlitz C, Zanetti Dällenbach R, Moffat R, Stiller R, Germeyer A, Nawroth F, Dangel A, Findeklee S, Tschudin S. Randomized controlled trial on the effect of an online decision aid for young female cancer patients regarding fertility preservation. Hum Reprod. 2019;34:1726-34. PMID: 31398258 DOI: 10.1093/humrep/dez136 Medline
Garvelink MM, ter Kuile MM, Bakker RM, Geense WJ, Jenninga E, Louwé LA, Hilders CG, Stiggelbout AM. Women’s experiences with information provision and deciding about fertility preservation in the Netherlands: ‘satisfaction in general, but unmet needs’. Health Expect. 2015;18:956-68. PMID: 23647741 DOI: 10.1111/hex.12068 Medline
Gonçalves V. Decisional Regret in Female Oncofertility Decision Making-An Integrative Narrative Review. Cancers (Basel). 2021;13:4735. PMID: 34638222 DOI: 10.3390/cancers13194735 Medline
Keim-Malpass J, Fitzhugh HS, Smith LP, Smith RP, Erickson J, Douvas MG, Thomas T, Petroni G, Duska L. What is the Role of the Oncology Nurse in Fertility Preservation Counseling and Education for Young Patients? J Cancer Educ. 2018;33:1301-5. PMID: 28667545 DOI: 10.1007/s13187-017-1247-y Medline
Kufel-Grabowska J, Podolak A, Maliszewski D, Bartoszkiewicz M, Ramlau R, Lukaszuk K. Fertility Counseling in BRCA1/2-Mutated Women with Breast Cancer and Healthy Individuals. J Clin Med. 2022;11:3996. PMID: 35887761 DOI: 10.3390/jcm11143996 Medline
Massarotti C, Lo Monaco L, Scaruffi P, Sozzi F, Remorgida V, Cagnacci A, Anserini P. Contraception in cancer survivors: insights from oncofertility follow-up visits. Gynecol Endocrinol. 2021;37:166-70. PMID: 32840160 DOI: 10.1080/09513590.2020.1810658 Medline
Müller M, Urech C, Boivin J, Ehrbar V, Moffat R, Daellenbach RZ, Rochlitz C, Tschudin S. Addressing decisional conflict about fertility preservation: helping young female cancer survivors’ family planning decisions. BMJ Sex Reprod Health. 2018;44:175-80. PMID: 29150522 DOI: 10.1136/bmjsrh-2017-101820 Medline
Oktay K, Harvey BE, Partridge AH, Quinn GP, Reinecke J, Taylor HS, Wallace WH, Wang ET, Loren AW. Fertility Preservation in Patients With Cancer: ASCO Clinical Practice Guideline Update. J Clin Oncol. 2018;36:1994-2001. PMID: 29620997 DOI: 10.1200/JCO.2018.78.1914 Medline
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. PMID: 27919275 DOI: 10.1186/s13643-016-0384-4 Medline
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. PMID: 33782057 DOI: 10.1136/bmj.n71 Medline
Peate M, Meiser B, Friedlander M, Zorbas H, Rovelli S, Sansom-Daly U, Sangster J, Hadzi-Pavlovic D, Hickey M. It’s now or never: fertility-related knowledge, decision-making preferences, and treatment intentions in young women with breast cancer--an Australian fertility decision aid collaborative group study. J Clin Oncol. 2011;29:1670-7. PMID: 21444865 DOI: 10.1200/JCO.2010.31.2462 Medline
Semler R, Thom B. Fertility Preservation: Improving Access Through Nurse-Advocated Financial Assistance. Clin J Oncol Nurs. 2019;23:27-30. PMID: 31538989 DOI: 10.1188/19.CJON.S2.27-30 Medline
Shen S, Zelkowitz P, Rosberger Z. Cancer and fertility: optimizing communication between patients and healthcare providers. Curr Opin Support Palliat Care. 2019;13:53-8. PMID: 30625120 DOI: 10.1097/SPC.0000000000000413 Medline
Speller B, Metcalfe K, Kennedy ED, Facey M, Greenblatt E, Scheer AS, Warner E, Joy AA, Wright FC, Baxter NN. The “Begin Exploring Fertility Options, Risks and Expectations” (BEFORE) decision aid: development and alpha testing of a fertility tool for premenopausal breast cancer patients. BMC Med Inform Decis Mak. 2019a;19:203. PMID: 31660965 DOI: 10.1186/s12911-019-0912-y Medline
Speller B, Sissons A, Daly C, Facey M, Kennedy E, Metcalfe K, Baxter NN. An evaluation of oncofertility decision support resources among breast cancer patients and health care providers. BMC Health Serv Res. 2019b;19:101. PMID: 30728004 DOI: 10.1186/s12913-019-3901-z Medline
Srikanthan A, Ethier JL, Amir E. The Voices of Young Women with Breast Cancer: Providing Support and Information for Improved Fertility Preservation Discussions. J Adolesc Young Adult Oncol. 2019;8:547-53. PMID: 31158039 DOI: 10.1089/jayao.2019.0030 Medline
Stacey D, Légaré F, Lewis K, Barry MJ, Bennett CL, Eden KB, Holmes-Rovner M, Llewellyn-Thomas H, Lyddiatt A, Thomson R, Trevena L. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4:CD001431. PMID: 28402085 DOI: 10.1002/14651858.CD001431.pub5 Medline
Takeuchi E, Kato M, Miyata K, Suzuki N, Shimizu C, Okada H, Matsunaga N, Shimizu M, Moroi N, Fujisawa D, Mimura M, Miyoshi Y. The effects of an educational program for non-physician health care providers regarding fertility preservation. Support Care Cancer. 2018;26:3447-52. PMID: 29681013 DOI: 10.1007/s00520-018-4217-y Medline
Taylan E, Oktay K. Fertility preservation in gynecologic cancers. Gynecol Oncol. 2019;155:522-9. PMID: 31604663 DOI: 10.1016/j.ygyno.2019.09.012 Medline
van den Berg M, Baysal Ö, Nelen WLDM, Braat DDM, Beerendonk CCM, Hermens RPMG. Professionals’ barriers in female oncofertility care and strategies for improvement. Hum Reprod. 2019;34:1074-82. PMID: 31111876 DOI: 10.1093/humrep/dez062 Medline
van den Berg M, van der Meij E, Bos AME, Boshuizen MCS, Determann D, van Eekeren RRJP, Lok CAR, Schaake EE, Witteveen PO, Wondergem MJ, Braat DDM, Beerendonk CCM, Hermens RPMG. Development and testing of a tailored online fertility preservation decision aid for female cancer patients. Cancer Med. 2021;10:1576-88. PMID: 33580749 DOI: 10.1002/cam4.3711 Medline
Whittemore R, Knafl K. The integrative review: updated methodology. J Adv Nurs. 2005;52:546-53. PMID: 16268861 DOI: 10.1111/j.1365-2648.2005.03621.x Medline
Wo JY, Viswanathan AN. Impact of radiotherapy on fertility, pregnancy, and neonatal outcomes in female cancer patients. Int J Radiat Oncol Biol Phys. 2009;73:1304-12. PMID: 19306747 DOI: 10.1016/j.ijrobp.2008.12.016 Medline
Zhao J, Liu J, Chen K, Li S, Wang Y, Yang Y, Deng H, Jia W, Rao N, Liu Q, Su F. What lies behind chemotherapy-induced amenorrhea for breast cancer patients: a meta-analysis. Breast Cancer Res Treat. 2014;145:113-28. PMID: 24671358 DOI: 10.1007/s10549-014-2914-x Medline
Zwingerman R, Melenchuk K, McMahon E, Liu KE, Siren A, Laferriere N, Greenblatt EM. Expanding Urgent Oncofertility Services for Reproductive Age Women Remote from a Tertiary Level Fertility Centre by Use of Telemedicine and an On-site Nurse Navigator. J Cancer Educ. 2020;35:515-21. PMID: 30820926 DOI: 10.1007/s13187-019-01490-w Medline